CASE REPORT
Coexistence of Competing Opportunistic Pathogens in Critically ill Patients with Advanced AIDS: A Case Report and Literature Review
Seregey Voznesenskiy1, Tatyana Ermak2, Karl Emerole1, *, Еlena Samotolkina3, Polina Klimkova3, Evgeniya Abramova3, Galina Kozhevnikova1
Article Information
Identifiers and Pagination:
Year: 2022Volume: 16
E-location ID: e187461362208040
Publisher ID: e187461362208040
DOI: 10.2174/18746136-v16-e2208040
Article History:
Received Date: 15/3/2022Revision Received Date: 15/4/2022
Acceptance Date: 20/5/2022
Electronic publication date: 27/09/2022
Collection year: 2022

open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Introduction:
Opportunistic infections (OIs) are the leading causes of morbidity and mortality among HIV-infected individuals. The incidence of OIs is greater in antiretroviral treatment (ART) naive patients. As of 30 June 2021, 28.2 (73%) people with HIV/AIDS (PLWHA) were accessing antiretroviral therapy (ART) globally, leaving the remaining 27% PLWHA without ART at risk for OIs. Multiple opportunistic infections are caused due to the coexistence of competing opportunistic pathogens that confound clinical manifestations, investigative procedures, and management protocols.
Case Presentation:
In this report, we describe the case of a critically ill HIV female patient admitted to the ICU. The patient was diagnosed with multiple opportunistic infections and subsequently died after her illness progressed. Due to the paucity of information on the subject, we conducted a retrospective study of 1440 case records of HIV/AIDS critically ill patients to determine the incidence and spectrum of multiple opportunistic infections. We performed a review of the available medical literature relevant to the subject.
Conclusion:
Knowledge of such events would guide and enhance the physician's diagnostic and management strategies, especially in resource limited regions.
1. INTRODUCTION
Opportunistic infections (OIs) are commonly identified in advanced HIV diseases and are considered a burden in countries with low antiretroviral therapy (ART) coverage. As of 30th June 2021, 28.2 (73%) people with HIV/AIDS (PLWHA) were accessing antiretroviral therapy (ART) globally, leaving the remaining 27% PLWHA without ART at risk for OIs [1]. In the Russian Federation, about 57% of the total number of people living with HIV/AIDS are receiving ART [2]. The spectrum and causative agents of OIs vary, and these pathogens exhibit different organ tropism. The synergistic and antagonistic interactions occurring during multiple co-infections or coexistence of competing opportunistic pathogens in HIV/AIDS are under-examined. These interactions between competing opportunistic pathogens can alter the immune response against subsequent infections by suppressing the immune system. Multiple opportunistic infections due to the coexistence of competing opportunistic pathogens confound clinical manifestations and investigative procedures. In addition, the multiple medications or polypharmacy used to treat these patients increases potential drug–drug interactions. The CD4 cell count and plasma HIV viral load are key prognostic markers for disease progression to AIDS or death among untreated HIV-infected individuals [3-9]. As the CD4 cell count falls below normal, and particularly once thresholds of 200 cells/µL or a CD4 percentage of 15% are reached, the risk of opportunistic infections and their coexistence rises [10]. Here, we report on an ART treatment-naive woman admitted to the intensive care unit (ICU) with advanced AIDS and multiple opportunistic infections, who has been followed from the initial diagnosis.
2. CASE PRESENTATION
A 44-year-old woman of Russian origin infected with HIV since 2015, with a past history of severe headache and esophageal candidiasis, presents to the Infectious Diseases Clinical Hospital No 2 in Moscow with lethargy, muscle pain and weakness. She was an antiretroviral-naive patient, and her history was remarkable for excessive alcohol consumption. Upon physical examination, neurologic examination revealed the patient to be disoriented to time, place, and person. She was responsive to verbal orders, with positive Kernig and Brudzinski signs. She appeared malnourished with loss of muscle mass and showed dysarthria with involuntary hand movement. Oral cavity inspection revealed whitish non erythematous pseudo membranous plaques on the hard palate, buccal surface of cheek and the floor of the mouth. Pulmonary auscultation revealed harsh breath sounds with crackles in the bases. At the time of admission, her CD4 cell count was 100 cells/ml and HIV RNA 848 176 copies/ ml. Routine blood tests yielded - hemoglobin of 9.2 g/dL, white blood count (WBC) of 2.5 × 109/L. The patient was investigated for opportunistic pathogens: Cytomegalovirus DNA (CMV-DNA) sequences was detected in the serum, bronchoalveolar lavage (BAL) and buccal swab samples; Blood cultures tested positive for fungal infection caused by Candida glabrata; BAL culture revealed the growth of C. glabrata and Klebsiella spp; A spinal tap yielded milky white color cerebrospinal fluid (CSF) and cryptococcal infection was confirmed by the detection of cryptococcal antigen; The CSF had an HIV viral load of 491042 copies/mL Brain computer tomography (CT) results demonstrated fluid accumulation in the subarachnoid space and hypodensities in the corona radiata. Chest radiography showed bilateral interstitial pneumonia Fig. (1). Chest CT identified moderate interstitial pulmonary edema and multiple bilateral lesions in both the lungs, triggering a suspicion of tuberculosis (TB) as we had observed similar chest CT findings in confirmed TB cases, though in this case, laboratory investigations did not confirm this opportunistic pathogen. Echocardiogram was performed and showed pericardial effusion, systolic and diastolic dysfunction, regional wall motion abnormalities, mitral valve vegetation, mild mitral and tricuspid regurgitation. Based on our investigations, a final diagnosis of Acquired Immune Deficiency Syndrome (AIDS), category C, based on the Centers for Disease Control (CDC) categorization, was made with the following opportunistic infections: HIV-associated neurological disorder; Disseminated cytomegalovirus infection; Pneumonia of mixed etiology (CMV, Kl. Pneumonia, C. glabrata); Disseminated candidiasis. The causative agent responsible for the cardiac manifestations (infective endocarditis with myocardial lesions, pericarditis) remained unclear. The patient received antibiotics, antimicotic (caspofungin, mycamine), anti-CMV (gancyclovir), and antiretroviral (abacavir/lamivudine, lopinavir/ritonavir) therapy. Despite treatment, the patient developed pancytopenia, with a progressive decreasing trend in platelet count and died due to cardiorespiratory failure and cerebral edema.
2.1. Case Records Review
Due to the paucity of information on the subject, we retrospectively reviewed 1440 case records of HIV/AIDS critically ill patients, to determine the incidence of multiple opportunistic diseases due to the coexistence of competing pathogens. These patients were admitted to the ICU between January 2018 and 2020 at the infectious disease clinical hospital Nº2, Moscow, Russia. About 900 (62.5%) patients were male and 540 were female (37.5%) with a mean age of 40 (SD ± 10.7) years, and most were diagnosed with CDC category C (87.6%). The median duration of HIV infection was 10.4 years (IQR 4.5–15.4 years). Their median CD4 count, and viral load was 100 cells/mm3 (IQR: 10-250) and 100,000 copies/ml (IQR: 50,000-500,000), respectively. 68% of the patients were ART treatment-naive. These patients had a median of 8 years (IQR 2.7–11.8years) without ART treatment at the time of presentation. We analyzed our data using STATA version 12 with a p-value ≤ 0.005 considered statistically significant. In total, 1007 (69.9%) coexistence of opportunistic pathogens were recorded, and 275 various patterns of these coexistence were identified. About 45.8% (462/1007) of patients had dual infections of opportunistic pathogens. The most common co-infection patterns recorded were a combination of bacterial pneumonia + unspecified encephalitis (15.7% of patients). It should be noted that most HIV/TB patients were transferred to a specialized TB hospital, therefore there was a low prevalence of TB (3.8%) in the reviewed cases. Patients with more cases of multiple opportunistic pathogens had a higher significant risk of death as shown in Table 1, while Table 2 illustrates the spectrum and prevalence of the coexisting opportunistic pathogens registered among the ICU HIV-infected patients.
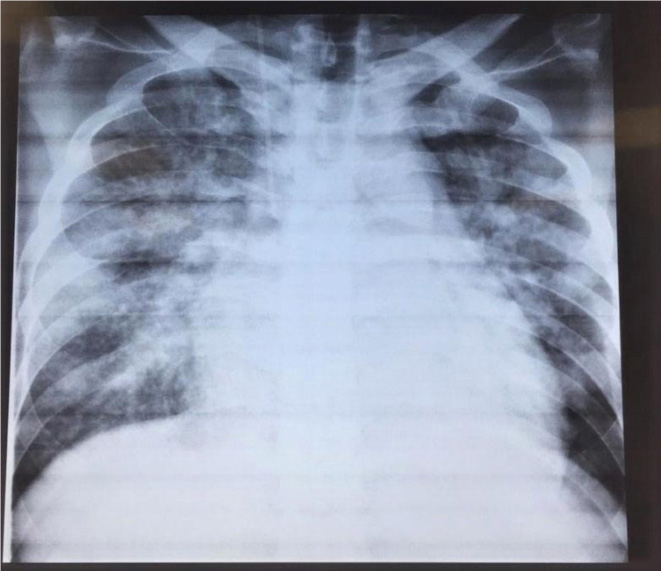 |
Fig. (1). Chest radiograph of the patient showing bilateral interstitial pneumonia upon admission to the intensive care unit. |
| NumbEr of Coexisting Opportunistic Pathogens |
Patients N (%) |
ICU Admission Outcome | P-value | |
|---|---|---|---|---|
| Death | TraNsferred to General Ward after Stabilization | |||
| 2 | 462 (45.8%) | 78.4% | 21.6% | <0.001 |
| 3 | 306 (30.3%) | 79.1% | 20.9% | <0.001 |
| 4 | 201 (19.9%) | 87.6% | 12.4% | <0.001 |
| 5 | 34 (3.3%) | 85.3% | 14.7% | 0.003 |
| 6 | 4 (0.3%) | 100% | 0% | |
| Total | 1007(100%) | 24.7% | 75.3% | <0.001 |
| Dual Infections | Patients N (%) |
|---|---|
| Bacterial pneumonia + Unspecified encephalitis | 159 (15.7%) |
| Bacterial pneumonia + Disseminated CMV infection | 45 (4.5%) |
| Bacterial pneumonia + Pneumocystis pneumonia | 32 (3.1%) |
| Bacterial pneumonia + Invasive candidiasis | 29 (2.8%) |
| Unspecified encephalitis + Disseminated CMV infection | 17 (1.6%) |
| Bacterial pneumonia + HIV encephalitis | 17 (1.6%) |
| Bacterial pneumonia + Neurotoxoplasmosis | 12 (1.2%) |
| Triple infections | Patients N (%) |
| Bacterial pneumonia + Unspecified encephalitis + Disseminated CMV infection | 39(3.8%) |
| Bacterial pneumonia + Disseminated CMV infection + Pneumocystis pneumonia | 31(3%) |
| Bacterial pneumonia + Disseminated CMV infection + Neurotoxoplasmosis | 14(1.3%) |
| Bacterial pneumonia + Unspecified encephalitis + Pulmonary candidiasis | 10 (0.9%) |
| Quadriple infections | Patients N (%) |
| Bacterial pneumonia + Disseminated CMV infection + Pneumocystis pneumonia + Invasive candidiasis | 34 (3.3%) |
| Bacterial pneumonia + Disseminated CMV infection + Neurotoxoplasmosis + Invasive candidiasis | 6 (0.6%) |
| Bacterial pneumonia + Unspecified encephalitis + Disseminated CMV infection + Invasive candidiasis | 3 (0.3%) |
| Quintuple infections | Patients N (%) |
| Bacterial pneumonia + Unspecified encephalitis + Disseminated CMV infection + Neurotoxoplasmosis+ Invasive candidiasis | 8 (0.8%) |
| Sextuple infections | Patients N (%) |
| Bacterial pneumonia + Unspecified encephalitis + Disseminated CMV infection + Pneumocystis pneumonia + Invasive candidiasis + Neurotoxoplasmosis | 1 (0.09%) |
| Bacterial pneumonia + Unspecified encephalitis + Disseminated CMV infection + Pneumocystis pneumonia + Invasive candidiasis + Kaposi sarcoma | 1 (0.09%) |
| Bacterial pneumonia + Unspecified encephalitis + Disseminated CMV infection + Pneumocystis pneumonia + Invasive candidiasis + Tuberculosis | 1 (0.09%) |
| Bacterial pneumonia + Unspecified encephalitis + Disseminated CMV infection + Invasive candidiasis + Mycobacterium Avium Complex infections + Lymphoproliferative disorders | 1 (0.09%) |
3. DISCUSSION
Only a few studies have described cases or the spectrum of multiple opportunistic infections, especially in advanced HIV diseases. Three large studies investigated the spectrums of opportunistic infections, malignancies, and severe comorbidities in HIV-infected patients [10-12]. They all found tuberculosis to be the most common OI and emphasized the relevance of the subject with respect to patient treatment and care. Exacerbations of HIV/AIDS comorbidities account for most ICU admissions in PLWHA [13]. There are no prospective evaluations of the safety, efficacy and timing of ART administration in the ICU. Clinicians frequently rely on expert opinion to guide decision-making regarding initiating, continuing, or stopping ART alongside managing critical illness. Individual case-by-case discussion with an infectious diseases/HIV specialist is recommended [13].
CONCLUSION
In conclusion, our case presentation and retrospective study which described a large cohort of HIV patients with multiple opportunistic, validate the importance of strategic timing of antiretroviral treatment (START) regardless of the geographical region. In addition, efforts to raise awareness of the spectrum of opportunistic infections including coexistence of the pathogens would guide and enhance the physician's diagnostic and management strategies, especially in resource limited regions. Such studies can also facilitate research on pathogens of unspecified origins.
LIST OF ABBREVIATIONS
| OIs | = Opportunistic Infections |
| ART | = Antiretroviral Treatment |
| PLWHA | = People with HIV/AIDS |
| WBC | = White Blood Count |
| CDC | = Centers for Disease Control |
| AIDS | = Acquired Immune Deficiency Syndrome |
| CSF | = Cerebrospinal Fluid |
| BAL | = bronchoalveolar lavage |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No animals/humans were used for studies that are the basis of this research.
CONSENT FOR PUBLICATION
Informed consent was obtained.
STANDARDS OF REPORTING
Care guidelines were followed.
AVAILABILITY OF DATA AND MATERIALS
The data of the study are available by contacting the corresponding author [K.E] upon reasonable request.
FUNDING
This paper has been supported by the RUDN University Strategic Academic Leadership Program.
CONFLICT OF INTEREST
The author declares no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The authors are grateful for the contribution of the Department of Intensive Care, Infectious Diseases Clinical Hospital No 2 Moscow. The authors are also grateful for the support of RUDN University Strategic Academic Leadership Program.
REFERENCES
| [1] | Global HIV & AIDS statistics — Fact sheet Retrieved from: https://www.unaids.org/en/resources/fact-sheet |
| [2] | Russia HIV & AIDS statistics — Fact sheet Retrieved from http://www.hivrussia.info/ |
| [3] | Jabs DA, Van Natta ML, Kempen JH, et al. Characteristics of patients with cytomegalovirus retinitis in the era of highly active antiretroviral therapy. Am J Ophthalmol 2002; 133(1): 48-61. |
| [4] | Lortholary O, Petrikkos G, Akova M, et al. ESCMID Fungal Infection Study Group. ESCMID guideline for the diagnosis and management of Candida diseases 2012: patients with HIV infection or AIDS. Clin Microbiol Infect 2012; 18(Suppl. 7): 68-77. |
| [5] | Schmidt W, Wahnschaffe U, Schäfer M, et al. Rapid increase of mucosal CD4 T cells followed by clearance of intestinal cryptosporidiosis in an AIDS patient receiving highly active antiretroviral therapy. Gastroenterology 2001; 120(4): 984-7. |
| [6] | Nightingale SD, Byrd LT, Southern PM, Jockusch JD, Cal SX, Wynne BA. Incidence of Mycobacterium avium-intracellulare complex bacteremia in human immunodeficiency virus-positive patients. J Infect Dis 1992; 165(6): 1082-5. |
| [7] | Sonnenberg P, Glynn JR, Fielding K, Murray J, Godfrey-Faussett P, Shearer S. How soon after infection with HIV does the risk of tuberculosis start to increase? A retrospective cohort study in South African gold miners. J Infect Dis 2005; 191(2): 150-8. |
| [8] | Wolff AJ, O’Donnell AE. Pulmonary manifestations of HIV infection in the era of highly active antiretroviral therapy. Chest 2001; 120(6): 1888-93. |
| [9] | Leonova ON, Stepanova YV, Belyakov NA. Severe and comorbid conditions in hiv patients: An analysis of adverse outcomes. Vichinfektsiia Immunosuppr 2017; 9(1): 55-64. [In Russ.]. |
| [10] | Mark W Hull, Marianne Harris, Julio S.G. Montaner. 102 - Principles of Management of HIV in the industrialized world.Infectious Diseases Fourth Ed. 2017; 912-7. |
| [11] | Xiao J, Gao G, Li Y, Zhang W, Tian Y, Huang Y. Correction: Spectrums of opportunistic infections and malignancies in HIV-infected patients in tertiary care hospital, China. PLoS One 2014; 9(1) |
| [12] | Shakhgildyan VI, Yadrikhinskaya MS, Safonova AP. Pattern of secondary diseases and current approaches to their laboratory diagnosis in patients with HIV infection. Epidemiology and Infec Dis 2015; 1: S24-30. |
| [13] | Barbier F, Mer M, Szychowiak P, et al. Management of HIV-infected patients in the intensive care unit. Intensive Care Med 2020; 46(2): 329-42. |








