All published articles of this journal are available on ScienceDirect.
Adjunct Therapy of Zinc Supplementation Increases Immunological Response in HIV-Infected Patients: A Systematic Review and Meta-Analysis
Abstract
Introduction:
Malnutrition greatly accelerates the impairment of immune function among HIV-infected patients. Zinc deficiency is often found in people living with HIV/AIDS, affecting their immune function. Several studies have evaluated the effect of zinc in HIV-infected patients, including CD4+ T-cells. However, the results have varied. This review aimed to evaluate the effect of zinc supplementation in HIV patients, particularly its effect on CD4+ T-cells count.
Methods:
Relevant publications were obtained from PubMed database, Google Scholar, COCHRANE, and Science Direct. The primary outcome was CD4+ T-cells count, while the secondary outcomes were viral load and zinc levels. Year of publication, type of study, population, doses of zinc given, duration of zinc administration, sample size, age, and baseline CD4+ T-cells counts were also obtained and reported. Quantitative data from the publications were analyzed using a fixed-effect model or a random-effect model.
Results:
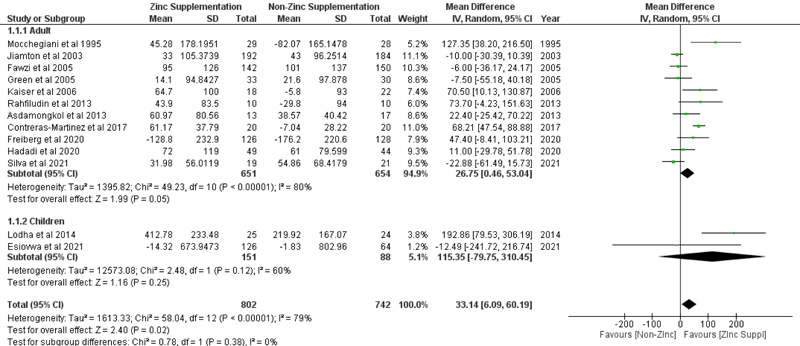
We evaluated 13 full-text articles on zinc supplementation in HIV-infected patients, involving 802 subjects for the experiment group and 742 subjects for the control group. Overall, zinc supplementation, whether as zinc supplementation-only or prepared as multiple micronutrient or multivitamin preparation, increases CD4+ T-cells counts by 33.14 cells/mm3 (p =0.02; 95% CI: 6.09 to 60.19), irrespective of age. Subgroup analysis revealed CD4+ T-cells counts also increase in patients who receive zinc supplementation-only preparation by 33.56 cells/mm3 (p = 0.04; 95% CI: 1.5 to 65.63). Zinc supplementation increases serum zinc levels with pooled mean difference of 15.41 µg/dl (p < 0.05; 95% CI: 12.77 to 18.06). However, the viral load did not significantly decrease with zinc supplementation, with a pooled mean difference of -4.02 copies/ml (p =0.7; 95% CI: -24.78 to 16.75), based on the random-effect model.
Conclusion:
Zinc supplementation in HIV-infected patients enhances immunological response, characterized by an increase in CD4+ T-cells counts. In addition, it increases zinc serum levels in HIV-infected patients, indicating the importance of zinc supplementation in this group of patients.
1. INTRODUCTION
Human Immunodeficiency Virus (HIV) is a retrovirus that primarily targets CD4+ T-cells and macrophages, subsequently leading to the suppression of CD4+ T-cells [1]. T cells are a key factor in reducing the impact of HIV infection; hence, their performance is greatly needed [2]. CD4+ T-cells can also be used as a severity indicator. CD4+ or CD8+ T-cells recognize viral antigenic peptides and may trigger various cytokines, e.g., interleukin-2 (IL-2), interferon- γ (IFN- γ), and tumor necrosis factor- β (TNF-β), subsequently triggering the multicellular immune response. Therefore, the lack of CD4+ T-cells as a consequence of HIV infection results in a decrement of CD8+ T-cells and cytokines [3].
Malnutrition remains a major concern for the adequate management of HIV-infected patients. It has been known that malnutrition accelerates the progression of HIV to acquired immune deficiency syndrome (AIDS). The condition is associated with the food adequacy of patients because it affects the ability to acquire, consume, and utilize food [4]. Studies have evaluated the epidemiological aspect of malnutrition in people living with HIV/AIDS. One study conducted in Brazil evaluated malnutrition prevalence in the 20-59 years old group. In the study involving 127 patients, malnutrition was found in 55 (43%) patients, defined by BMI < 18.5 kg/m2. A total of 15% were in a severe malnutrition state, indicated by a BMI of < 16 kg/m2 [5].
The magnitude of malnutrition in HIV-infected patients affects HIV-infected patients' mortality and morbidity rate. The effect of malnutrition on mortality and morbidity is supported by the Nutrition for Healthy Living (NFHL) study. The risk of death increases by 11% for every 1% increase in weight loss since the previous visit. The risk might increase sixfold if weight loss was >10% below the baseline weight [6].
Malnutrition conditions affect susceptibility to infection by altering the immune system in various possible ways. The weakened immune status results in an increment of HIV replication, consequently accelerating HIV progression to AIDS. Furthermore, untreated patients with HIV/AIDS are at risk for malnutrition. A similar outcome also happens in infants and children (less than five years old) [7, 8].
Among the malnutrition in HIV-infected patients, zinc deficiency is often found. It may be associated with a decrease in immunity to maintain the body's immune systems. A study by Baum et al. [9] reported zinc deficiency occurs in HIV-infected adults reached >50%. The result is consistent with the study by Asemota et al. [10], which found that 56% of 100 HIV patients had zinc deficiency.
The impact of zinc deficiency includes the detain in the metabolism of nutrients. A low level of zinc in plasma may reduce the sensitivity of the immune system to various infections. It also weakens the activity of phagocytosis and the production of cytokines [11]. Inadequate amounts of cytokines, primarily T helper (Th) cells, interferon (IFN), and leucocytes together with B lymphocytes, may alleviate pathogens invading the body's immune system.
Various randomized controlled trials (RCTs) have investigated the effect of zinc supplementation on HIV patients [12-24]. The outcomes included: CD4+ T-cells counts, viral load, neuropathy, diarrhea, and death. Considering the effect of zinc supplementation toward CD4+ T-cells counts, varying results were obtained. To the authors’ knowledge, only three studies closest to the meta-analysis of zinc supplementation toward CD4+ T-cells counts in HIV-infected patients were available. Zeng et al. [25] conducted a systematic review in 2011 on the effects of zinc supplementation in adult patients, children, and pregnant women. However, the meta-analysis that specifically analyzes zinc's effect on CD4+ T-cells counts only included three studies. Furthermore, there are ten years of gap to date, and other RCTs have been carried out with various results obtained. Kayode and Anaba [26] conducted a systematic review study regarding vitamin D, selenium, or zinc supplementation in patients with HIV. They discussed the effect of zinc on various outcomes in HIV-infected patients, e.g., CD4+ T-cells, diarrhea morbidity, zinc levels, and others. However, no meta-analysis was done. Jiang et al. [27] conducted a meta-analysis but focused on micronutrient supplementation; the study did not specifically address zinc and involved only six studies without evaluating CD4+ T-cells counts. Therefore, further studies are needed to evaluate the effect of zinc supplementation in HIV patients, especially its effect on CD4+ T-cells cell count. This review aimed to evaluate the effect of adjunct therapy of zinc supplementation on the immunological response of HIV-infected patients, characterized by the change in CD4+ T-cells count.
2. MATERIALS AND METHODS
2.1. Literature Search
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement guidelines were followed for conducting and reporting meta-analysis data. The eligibility criteria were decided by implementing the patient, intervention, comparison, and outcome (PICO) concept. The framework of PICO used for this study is depicted in Table 1. The eligibility criteria (PICO) were extracted into keywords utilizing the Boolean operator. In this study, we used keywords (HIV OR human immunodeficiency virus) AND (zinc) AND (CD4 OR Cluster of differentiation 4) AND (RCT OR Randomized controlled trial) in PubMed database, Google Scholar, COCHRANE, and Science Direct as a search engine to find eligible journals. As for Google Scholar, we screen the first 200 relevant articles from a total of 21,700 articles [28]. We also evaluate the references of the relevant articles. The last search was run on May 7th, 2021.
2.2. Study Selection
The study selection process was conducted by three authors (DAS, IKAS, KS) to minimize the likelihood of ruling out potentially relevant studies. When disagreement took place, the decision of the first, second and third authors was considered. Study selection started with disposing of duplicate records. Title and abstract screening were performed to exclude irrelevant studies. Subsequently, studies that passed the first evaluation were further assessed to evaluate their compliance with the inclusion and exclusion criteria for this review. All studies included were thoroughly evaluated for their quality before eventually being included by implementing the critical appraisal tool of Cochrane Collaboration’s risk-of-bias method [29].
The current systematic review and meta-analysis included all studies prior to May 7th 2021, with full-text available, evaluating the additional administration of zinc in HIV-infected patients. Article with the type of case report, qualitative and economic studies, review, cadaveric and anatomic was excluded from the current study. All articles that did not provide the required data for conducting a meta-analysis were also excluded. To prevent duplication, articles written by the same author within the same institution were evaluated for the subjects.
| Patient | Human immunodeficiency virus |
| Intervention | Zinc supplementation |
| Comparator | Placebo |
| Outcome | CD4+ T-cells |
2.3. Data Extraction
This review included studies with HIV-infected patients who received zinc supplementation, whether as multiple micronutrient supplementation or zinc-only preparation. We categorized according to age (adult or children) and zinc preparation (zinc supplement only preparation or multiple micronutrient/multivitamin preparation). The immunological response was assessed with CD4+ T-cells counts in cells/mm3. The reviewed intervention is zinc supplementation, whether as multiple micronutrient supplementation or zinc-only preparation. We included studies that administered zinc for any doses, delivery method, and duration. However, we noted both the doses and the duration of zinc supplementation given for each study, along with the preparation and age of the study subjects.
The primary outcome investigated in the current systematic review and meta-analysis was the CD4+ T-cells count (cells/mm3) as the immunological response. The secondary outcome included viral load (copies/ml) and zinc serum level (µg/dl). All authors used an electronic data collection form to collect the required data from each article. The data was then combined and managed with software Review Manager 5.4.
The data items were the author’s name, year of publication, type of study, population, doses of zinc given, duration of zinc administration, sample size, age, and baseline CD4+ T-cells counts. The mean difference of CD4+ T-cells, viral load, and zinc serum level after and before administration of zinc in both treatment and control groups were performed the meta-analysis.
2.4. Risk of Bias
A standardized critical appraisal tool was utilized to ensure the quality of all articles that complied with the eligibility criteria for this review. This process, which aimed to minimize the likelihood of bias in study selection, was performed independently by two authors (DAS and KTPM). The critical appraisal tool employed for this review was Cochrane Collaboration’s risk-of-bias method [29].
2.5. Statistical Analysis
The mean difference of CD4+ T-cell counts in HIV-infected patients with zinc supplementation and without zinc supplementation were pooled and analyzed, along with the funnel plot. The analysis was also done as subgroups, stratified by age (adult and children) or zinc preparation (zinc supplementation only and received zinc as multiple micronutrient or multivitamin preparation). If data were presented as median with Q1 and Q3, the mean was calculated using a calculator, along with standard deviation (SD) and standard error (SE). We also combined two treatment groups in a specific article into one group, according to its allocation (treatment or control), by using the calculator. Heterogeneity was analyzed with Tau2, Chi2 or I2. The fixed-effect model (FEM) or random effect model (REM) was used accordingly. Meta-analysis was performed with software Review Manager 5.4.
3. RESULTS
3.1. Study Selection
By utilizing the foremost search strategy, we found a total of 250 studies from database searching and no additional records identified through other sources. After the duplicates were removed, we obtained 214 articles. We excluded 182 articles by screening the titles and abstracts, leaving us 32 relevant studies. Studies that did not provide all the information needed for this meta-analysis, a report from the same study, and studies in which the comparator group without zinc was not available were excluded. Screening and qualitative evaluation were performed; then, we obtained 13 articles used for the current systematic review and meta-analysis. PRISMA study flow diagram is depicted in Fig. (1).
3.2. Study Characteristics
We included 13 full-text articles on zinc supplementation in HIV-infected patients. The publication year of these articles varied from 1995 to 2021, with a total of 802 subjects for the experiment group and 742 subjects for the control group. The experiment group received zinc supplementation in varying doses and duration. All patients in the studies included were in antiretroviral treatment, except for the study by Esiovwa et al. [12], Freiberg et al. [14], and Jiamton et al. [23]. However, the majority (94.7%) of the subjects in the study by Esiovwa et al. [12] were on highly active antiretroviral therapy (HAART). In the study by Freiberg et al. [14], no important differences in ART initiation occurred between the experiment and control group. As for the study by Jiamton et al. [23], only 10 participants of the total participants were taking antiretroviral (five in the experiment group and five in the control group; the total participants in the experiment group were 192 subjects and the control group had 184 subjects). The summary of findings and studies characteristics is depicted in Table 2.

| Author | Type of Study | population | Zinc intervention and comparator | Duration | Sample Size, experiment vs. control | Age, experiment vs. control | Baseline CD4 T-cells, experiment vs control | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Esiovwa et al. (2021) [12] | Double-blind, randomized controlled study | HIV-positive children, aged 5-12 years | Experiment: multivitamin with zinc 5 mg. Control: multivitamin without zinc |
6 months | 126 subjects vs. 64 subjects | 7.75 ± 1.94 and 8.08 ± 1.94 vs. 8.08 ± 1.99 years old | 971.27 ± 541.17 vs. 975.44 ± 453.07 cells/µL | |||
| Silva et al. (2021) [13] | Two-stage of study. The first stage is case and controls, paired by age and sex in a 1:1 ratio. The second stage is randomized patients to receive intervention versus a placebo | Confirmed HIV with immunovirological discordance (IVD) patients | Zinc sulfate at a dose of 15 mg/day versus placebo | 12 months | 19 subjects vs. 21 subjects | 46.0 (40.0-54.0) vs. 48 (44-55.0) years olda | 197 (177-216) vs. 180 (131-198) cells/mm3a | |||
| Freiberg et al. (2020) [14] | double-blinded placebo-controlled randomized clinical trial | HIV-infected patients | Experiment: zinc gluconate capsule and 50 mg of riboflavin (adherence measure). Men: 15 mg of elemental zinc gluconate and women: 12 mg daily by mouth Control group: sucrose placebo with riboflavin |
18 months | 126 subjects vs. 128 subjects | 34 ± 5 vs. 34 ± 6 years old | 511 ± 296, vs 530 ± 288 | |||
| Hadadi et al. (2020) [15] | Randomized double-blind placebo-controlled trial | Adult patients (18 and 60 years) with confirmed HIV-1 infection, who were receiving combination antiretroviral therapy (CART) | Experiment: 220 mg zinc sulfate capsules (= 50 mg of elemental Zn) once daily (before lunch). Control: placebo capsules | 6 months | 49 subjects vs 44 subjects | 38.1 (8.8) vs 38.6 (8.8)b | 316 (±66) vs 312 (±68) cells/mm3b | |||
| Contreras-Martinez et al. (2017) [16] | A double-blind, randomized, placebo-controlled clinical trial | HIV-infected patients | Experiment: A daily dose of 20 mg of zinc sulfate. Control: placebo |
12 weeks | 20 subjects vs 20 subjects | 50.2 ± 9.7 vs 48.7 ± 9.4 years old | 157 (86.75) vs control: 163 (56)a | |||
| Lodha et al. (2014) [17] | Randomized, double-blind, placebo-controlled trial | All HIV-1-infected children older than 6 months in whom initiation of ART was indicated as per the guidelines | Experiment: 20 mg of elemental zinc as sulfate daily for 24 weeks Control: Placebo. Both groups received 1 recommended dietary allowance (RDA) of multivitamins everyday. |
24 weeks | 25 subjects vs 24 subjects | Mean (SD): 80.4 ± 43.6 vs 86.6 ± 50.6 months | 412.5 (196.5-645) vs 392.5 (269.25-874.25) cells/µla | |||
| Asdamongkol et al. (2013) [18] | A pilot clinical study of two phases; phase one was a cross-sectional study, and phase two was a prospective, randomized, placebo-controlled clinical trial | HIV-infected patients with immunological discordance | Experiment: Daily zinc supplementation consisted of 15 mg of chelated zinc. Control: Placebo |
Six months | 13 subjects vs 17 subjects | 45.0 ± 11.0 years old in total. No data for intervention and control group | 183 (151-213) vs 162 (139-182) cells/mm3a | |||
| Rahfiludin et al. (2013) [19] | Experimental study with pre-test post-test with control group design | HIV-infected patients | Experiment: Zinc supplementation 5mg/day, along with AZT. Control: Only AZT |
One months | 10 subjects vs 10 subjects | Not clear | 371.3 ± 126.8 vs 396.3 ± 257.9 cells/µL | |||
| Kaiser et al. (2006) [20] | Prospective, randomized, double-blinded, placebo-controlled clinical trial | HIV-infected patients | Experiment: Micronutrient (include zinc 30 mg) supplement taken twice daily. Control: Placebo. |
12 weeks | 18 subjects vs 22 subjects | 45.6 ± 7.81 vs 46.6 ± 6.95 years old | 357 ± 154 vs 467 ± 262 cells/µg | |||
| Fawzi et al. (2005) [21] | A double-blind, randomized controlled trial | Pregnant women, between 12 and 27 weeks of gestation, who were HIV-infected | Experiment: 25 mg Zn as zinc sulfate included in an effervescent tablet, one tablet in water every day. Control: Placebo |
Between recruitment and 6 weeks after delivery | 142 subjects vs 150 subjects | 26.7 ± 4.9 vs 27 ± 5.0 years old | 401 ± 203 vs 415 ± 210 cells/mm3 | |||
| Green et al. (2005) [22] | A double-blind, randomized, placebo-controlled trial | Male and female HIV-positive patients. | Experiment: 220 mg of zinc sulphate, equivalent to 50 mg of elemental zinc and, as one capsule daily Control: placebo once daily for the same duration. |
28 days | 33 subjects vs 30 subject | 40 (7.8) vs 40 (8.3)c | 113.1 (60.2) vs 134.3 (63.1) cells/µlc | |||
| Jiamton et al. (2003) [23] | Randomized placebo-controlled trial | HIV-infected men and women | Experiment: multivitamin and mineral contained zinc 30 mg, one tablet twice a day after food Control: Placebo tablets contained dibasic calcium phosphate and were coated with iron oxide. |
48 weeks | 192 subjects vs 184 subjects | 32 (18, 63) vs 32 (20, 60) years oldd | 244 (52-544) vs 261 (50-550) cells x 106/le | |||
| Mocchegiani et al. (1995) [24] | Not clearly described | Stage III and stage IV of HIV-infected patients | Experiment: Zinc sulphate given orally at a dose of 200 mg/day (=45.5 mg Zn2+). Control: not stated |
One months | 29 subjects (17 subjects for stage III + 12 subjects stage IV C1) vs 28 subjects (18 subjects for stage III + 10 subjects for stage IV C1) | Not clear | 80 ± 10 (stg IV C1) and 325 ± 18 (stg III) vs 74 ± 11 (stg IV C1) and 348 ± 19 (stg III) cells/mm3 | |||
aData presented as median (IQR), bData presented as mean (SE), cData presented as mean (SD), dData presented as mean (range), eData presented as median (range)
HIV: Human immunodeficiency virus, SD: Standard deviation, SE: Standard error, IQR: Interquartile range.
3.3. Risk of Bias within Studies
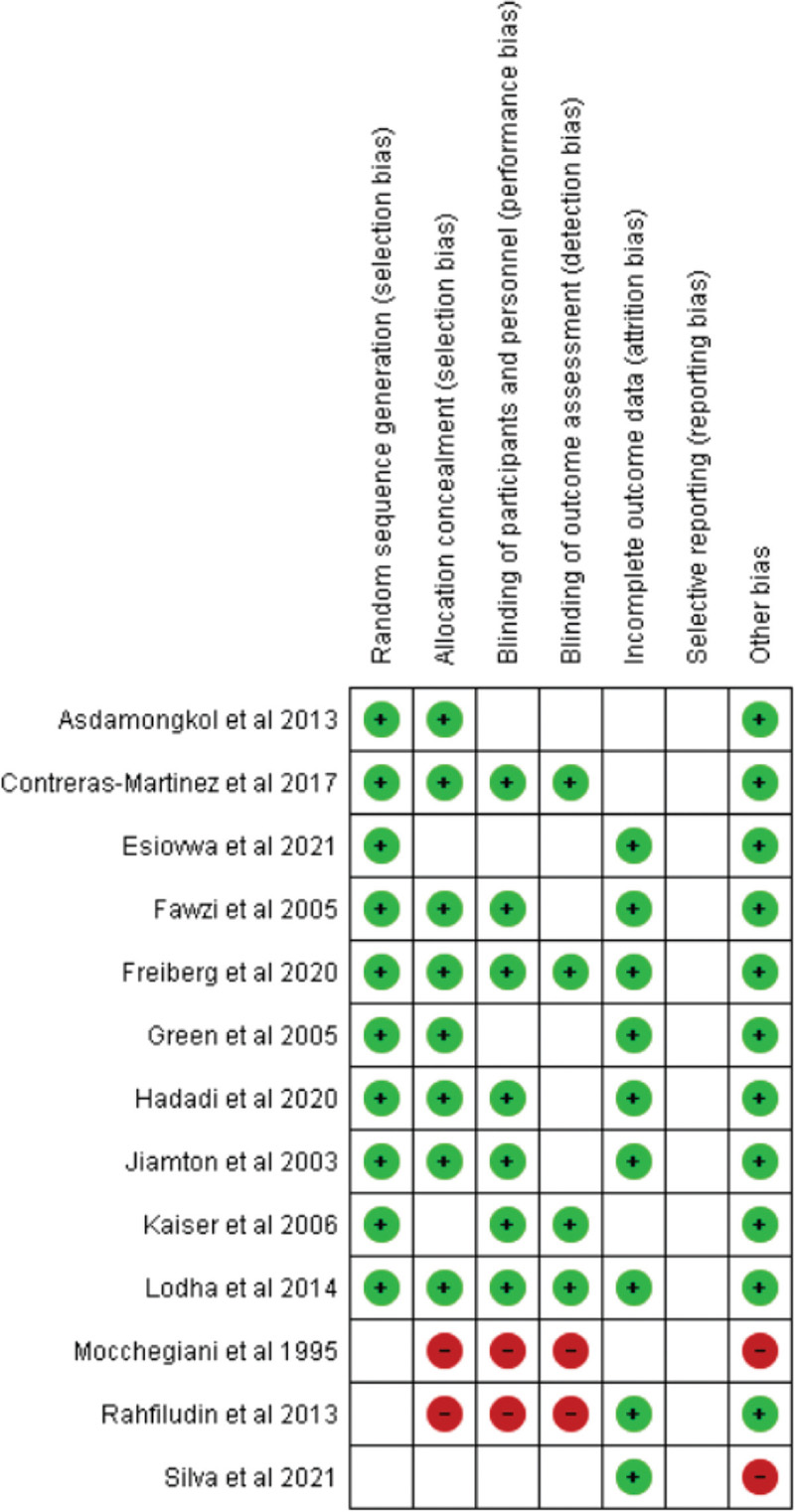
The risk of bias was analyzed using Cochrane Collaboration’s risk-of-bias method. All 13 articles included in the current study were evaluated for quality. The complete result of the risk of bias for all included articles is described in Fig. (2).
3.4. Effect of Zinc Supplementation on CD4+ T-cells Counts of HIV-Infected Patients
Overall, zinc supplementation, whether as zinc supplementation-only or prepared as multiple micronutrient or multivitamin preparation, may improve immunological response in HIV-infected patients by increasing CD4+ T-cells counts. Based on random effect model (I2=79%; χ2 = 58.04; p < 0.00001), pooled mean difference of CD4 T-cells counts between zinc supplementation and without zinc supplementation was 33.14 cells/mm3 (p =0.02; 95% CI: 6.09 to 60.19) (Fig. 3), indicating that zinc supplementation increases CD4+ T-cells counts significantly by 33.14 cells/mm3. Even though subgroup analysis according to age did not find that zinc supplementation significantly increases CD4+ T-cells counts (p = 0.05 for adults and p = 0.25 for children), pooled analysis found a significant increase of CD4+ T-cells in zinc treated group. Funnel plot analysis is depicted in Fig. (4).
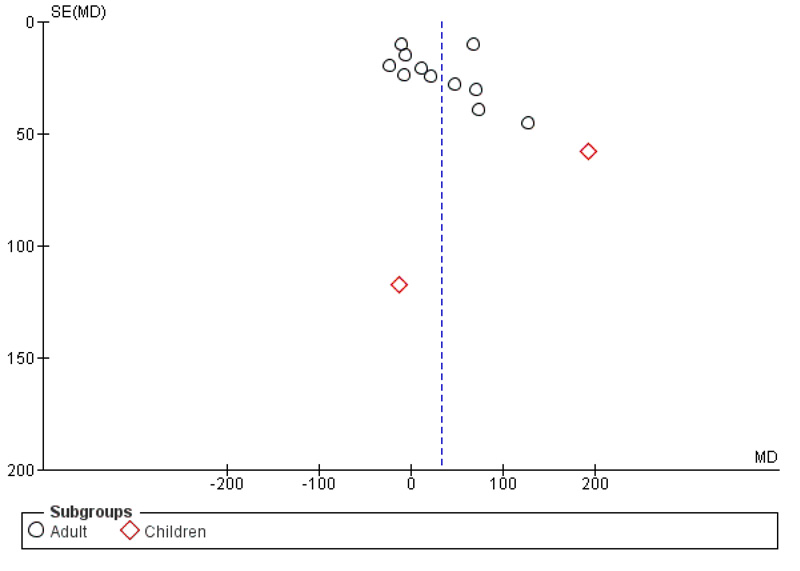
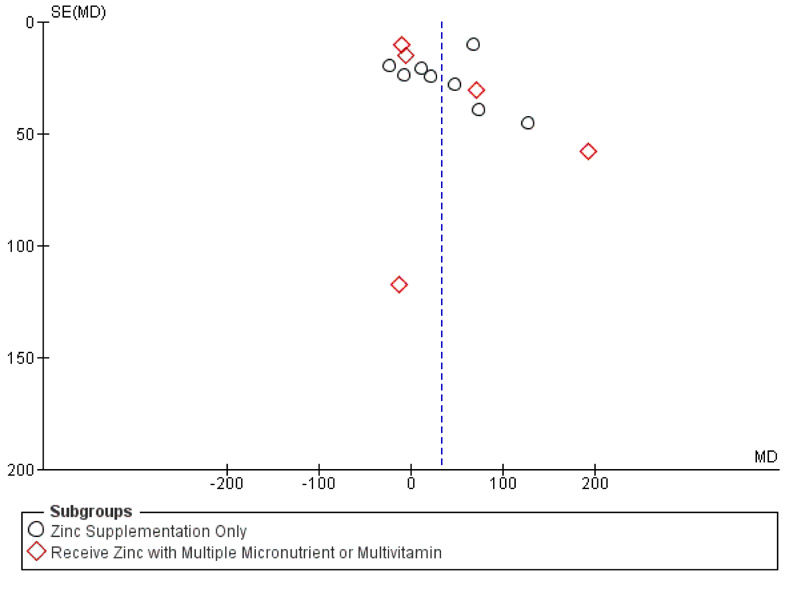
To determine whether the effect of the increase in CD4+ T-cells counts was due to other effects of micronutrients/multivitamins or not, we subgrouped the analysis according to the intervention given (zinc supplementation only or receiving zinc as multiple micronutrient or multivitamin preparation). According to subgroup analysis, CD4+ T-cells counts also increase in patients who receive zinc supplementation only preparation by 33.56 cells/mm3 (p = 0.04; 95% CI: 1.5 to 65.63), with random effect model analysis (Fig. 5). Funnel plot analysis is depicted in Fig. (6).
3.5. Effect of Zinc Supplementation on Viral Load and Serum Zinc Level of HIV-Infected Patients
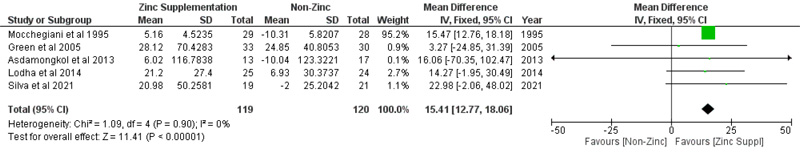
The pooled mean difference of viral load, which included three studies, after zinc supplementation was -4.02 copies/ml (p =0.7; 95% CI: -24.78 to 16.75), based on the random effect model (Fig. 7), meaning viral load did not significantly decrease with zinc supplementation. Meanwhile, the effect of zinc supplementation on serum zinc levels showed a significant increase in serum levels. Zinc supplementation increases serum zinc levels with a pooled mean difference of 15.41 µg/dl (p < 0.00001; 95% CI: 12.77 to 18.06) (Fig. 8), based on fixed effect model analysis.


4. DISCUSSION
The current meta-analysis highlighted the importance of zinc supplementation in HIV-infected patients. Overall, zinc supplementation, whether as zinc supplementation only or prepared as multiple micronutrient or multivitamin preparation, may improve immunological response in HIV-infected patients by the increase in CD4+ T-cells counts irrespective of age or the preparation. Furthermore, considering the preparation given, zinc supplementation-only preparation also increases CD4+ T-cells count.
The main finding in the current meta-analysis was zinc supplementation increases CD4+ T-cells counts significantly by 33.14 cells/mm3. Considering the fact that the immune system in the body is greatly influenced by zinc deficiency, the increase of CD4+ T-cells in people with HIV/AIDS by the addition of zinc supplementation offers treatment benefits. Immunological failure is one of the criteria for determining treatment failure in people with HIV. It is defined by a fall of CD4 T-cells counts to pretherapy baseline (or below) or a 50% fall from the on-treatment peak value (if known) or persistent CD4 T-cells levels below 100 cells/mm3 six months after ART initiation [30]. Therefore, the CD4+ T-cell count is an issue that every clinician should be wary of. The increase in CD4+ T-cell count by 33.14 cells/mm3 by adding zinc supplementation offers a clinical advantage.
It has been known that other micronutrients and multivitamins also affect the immune system of the body [31]. In order to understand whether the effect of the increase in CD4+ T-cells count is due to other effects of micronutrients/multivitamins or not, this meta-analysis divided the subgroups according to the intervention given. The intervention is divided into zinc supplementation only or receiving zinc as multiple micronutrients or multivitamin preparation. According to subgroup analysis, CD4+ T-cells counts also increase in patients who receive zinc supplementation only preparation.
The exact mechanism of zinc in regulating various processes of immune response is still not completely elucidated. Zinc is a chemo-attractant for several immune cells, and its lack of amount may result in the decrease of polymorphonuclear cells’ chemotaxis [32]. A decrease in zinc concentration also hampers phagocytosis; hence its supplementation increases phagocytosis. In the adaptive immune system, zinc deficiency affects T cells, either in the developmental stage or functionally. An inadequate amount of zinc leads to atrophy of thymic and T-cell lymphopenia [33]. Furthermore, zinc is responsible for T-cell activation by stimulating the autophosphorylation of the protein kinase Lck via interaction with the cytoplasmic tails of CD4 and CD8 T-cells [34].
Several RCT studies have been conducted on whether zinc supplementation may improve the outcome in HIV-infected patients, including neuropathy, infection, and death. Considering its effects on CD4+ T-cells counts, varying results were obtained [12-24]. However, the studies that specifically analyzed the effect of zinc supplementation on CD4 T-cell changes are scarce in the literature. Compared to the current study, only three studies closest to the meta-analysis of zinc supplementation toward CD4+ T-cells counts in HIV-infected patients were available. However, the limitations of each study restrict the applicability of the results obtained to the clinical setting. The current study addressed the limitations of the previous studies. A total of 13 articles were included in this study. We subgrouped it according to age (adults and children) and zinc preparation (zinc-only and zinc as micronutrients or multivitamins added) to evaluate its effect specifically for adults or its preparation.
Considering that the increase in CD4+ T-cells cells count was influenced by antiretroviral therapy, we evaluate the use of antiretroviral therapy for this review. Three studies mixed the subjects with antiretroviral therapy and without antiretroviral therapy [12, 14, 23]. In one study, the majority (94.7%) of the subjects were on highly active antiretroviral therapy (HAART) [12]. Another study showed no important differences in ART initiation between the experiment and control group [14], while in the other study [23], only a small portion of participants was taking antiretroviral (five in the experiment group and five in the control group; the total participant in experiment group was 192 subjects and control group was 184 subjects). Considering those facts, it could be assumed that little or no influence of antiretroviral therapy in CD4+ T-cells count changes in the current meta-analysis.
The current meta-analysis is also subject to limitations. This study focused primarily on CD4+ T-cells counts, even though the secondary outcome also targeted its effect on viral load. A further study analyzed its effect on viral load, and more studies should be performed in the future. Additionally, as a small portion of subjects were distinct in relation to the use of HAART or not, a small likelihood should be considered, as it may affect the outcome of zinc supplementation on CD4+ T-cell count or viral load. Various durations of zinc supplementation, different HAART regimens and durations, and adherence would influence the outcomes. Furthermore, it would also be beneficial to evaluate the effect of zinc supplementation on other parameters, e.g., comorbidity incidence or other opportunistic infections.
CONCLUSION
Zinc supplementation increase CD4+ T-cells counts of HIV-infected patients, irrespective of the age of the patients or the preparation of zinc administered to the patients. It also increases serum zinc levels of HIV-infected patients, indicating the importance of zinc supplementation for HIV-infected patients.
CONSENT FOR PUBLICATION
Not applicable.
STANDARDS OF REPORTING
PRISMA guidelines have been followed.
AVAILABILITY OF DATA AND MATERIALS
The data of the study would be made available by contacting the corresponding author [K.A.S] on reasonable request.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.
SUPPLEMENTARY MATERIAL
PRISMA checklist is available as supplementary material on the publisher’s website along with the published article.


