All published articles of this journal are available on ScienceDirect.
Association between Higher CD32a+CD4+ T Cell Count and Viral Load in the Peripheral Blood of HIV-infected Patients
Abstract
Background:
The significance of CD32a receptor expression in individuals infected with Human Immunodeficiency Virus (HIV) is currently unclear. Previously, B. Descours et al. (2017) concluded that in patients infected with HIV-1, CD32a is expressed on resting T cells that contain HIV DNA. According to the authors, these cells are reservoirs for inducible, replication-competent viruses. However, other studies have reported that CD32a expression is associated with activated T cells and is not a marker of HIV-1 reservoirs. The aims of this study were: to determine the significance of the CD32a marker in HIV infection, to assess its expression on T helper (Th) subpopulations in peripheral blood of HIV-infected individuals and to clarify the relationship between this expression and viral load.
Methods:
For comparative analysis, the following groups were used: 27 HIV-infected patients; 11 individuals with Hepatitis C Virus (HCV) infection; 16 individuals with Hepatitis B Virus (HBV) infection; and 13 healthy donors. Peripheral blood served as the study material. The expression of CD32a receptor on Th cell subpopulations was assessed using flow cytometry. Nonparametric statistical methods were used for data analysis.
Results:
It was found that relative CD32a+ Th cell counts in HIV-infected individuals significantly exceeded corresponding values in other groups: healthy individuals (p<0.0001), those with HCV infection (p=0.0008) and those with HBV infection (p <0.0001). Among the Th subpopulations in HIV-infected patients, the CD32a receptor was predominantly expressed on Th1 cells (p<0.0001) and Th2 cells (p<0.0001), compared with Th17. We found a strong, direct correlation (r=0.78; p<0.0001) between viral load and CD32a+CD4+ T cell count in peripheral blood of HIV-infected individuals.
Conclusion:
Thus, our results provide evidence that the CD32a receptor can serve as a marker of HIV infection, and its expression depends on viral load. Clinical material was used here, for the first time, to show that CD32a is predominantly expressed on Th1 and Th2 cells.
1. INTRODUCTION
Currently, Human Immunodeficiency Virus (HIV) remains a global healthcare problem. Although Antiretroviral Therapy (ART) is effective, HIV still persists in the bodies of patients undergoing therapy and can rapidly replicate if treatment is interrupted. Thus, patients require life-long ART. The search for HIV reservoirs, including specific biomarkers of latently infected cells, is an urgent task of modern medicine. It is possible that such markers could be potential therapeutic targets for targeted killing of the virus in infected cells. In 2017, research by Descours B. et al. showed that resting CD4+ T cells containing HIV DNA express the CD32a receptor on their surface [1]. They claimed that the CD32a receptor on T helper cells is a biomarker of latently infected cells that are reservoirs of infection.
CD32a is a low-affinity Fc receptor for IgG. It falls into the human FcγRII family (also known as CD32 in the cluster of differentiation nomenclature) that primarily consists of cell membrane receptor proteins. Proteins of this family have high homology in their amino acid composition. They are encoded by three highly related genes (FCGR2A, FCGR2B, FCGR2C), which arose by recombination of the FCGR2A and FCGR2B genes [2]. According to the FcγR classification, there are activation and inhibitory receptors. Activation receptors include FcγRIIa (CD32a) and FcγRIIc (CD32c), while FcγRIIb (CD32b) is an inhibitory receptor. The ratio of these receptors on a cell determines its activation threshold [3]. All FcγRII receptor isoforms are low-affinity receptors that poorly interact with monomeric IgG, but can bind multivalent IgG complexes, such as immune complexes, with high affinity.
The expression and function of type II FcγR have been extensively researched for cells of innate immunity and B lymphocytes [4-6]. However, the expression and function of CD32 on CD4+ T cells remain unclear. Some studies have shown that CD4+ T cells do not express FcγRs [7, 8]. Other studies have found that from one to five percent of CD4+ T cells express the CD32 receptor on their surface [9, 10].
Currently, the significance of the CD32a marker in HIV infection is unclear. Previous studies by Descours B. et al. (2017) concluded that in patients infected with HIV-1, CD32a is expressed on resting T cells that contain HIV DNA [1]. According to the authors, these cells are reservoirs for the inducible, replication-competent virus. Later, Darcis et al. (2020) showed that CD32 remains a promising candidate marker of the HIV reservoir [11]. A new, optimized protocol for purification of CD4+CD32+ T cells was used, and it was shown that these cells are highly enriched for HIV DNA. Moreover, HIV proviruses in purified CD4+CD32+ T cells can be reactivated ex vivo to produce virus. However, other studies have reported that CD32a expression is associated with activated T cells and is not a marker of HIV-1 reservoirs [10, 12-14]. This leaves several open questions. Is the CD32a receptor a marker of resting T helper cells acting as HIV reservoirs? Is it associated with activated or non-activated cells? Does its expression depend on viral load or therapy?
As all members of the FcγRII family are integral membrane glycoproteins and contain conserved extracellular domains (featuring 85% amino acid homology) [15], analysis of receptor function faces difficulties when using monoclonal antibodies. In the majority of previous studies, CD32 expression was analyzed with the use of a pan-CD32 antibody capable of binding both the CD32a and CD32b isoforms. In addition, many in vitro experiments have used: density gradients for isolation of peripheral blood mononuclear cells (PBMCs); freezing and thawing of cells; and/or magnetic separation of CD32+ cells. All of these factors may affect CD32a surface receptor expression analysis of T helper cells.
The purposes of this study were to determine the significance of the CD32a marker in HIV infection, to assess its expression on T helper subpopulations in peripheral blood of HIV-infected individuals, and to clarify the relationship between this expression and viral load.
2. MATERIALS AND METHODS
2.1. Patients
For this study, 27 HIV-infected patients (Table 1) registered with the Leningrad Regional Center on Prevention and Control of AIDS and Infectious Diseases were examined. HIV diagnoses were confirmed by (positive results using) electrochemiluminescence immunoassay (ECLIA), which detects HIV-specific antibodies and p24 antigen, and by Western blot analysis. Clinical profiles of HIV-infected individuals were completed according to the HIV infection classification currently in effect in the Russian Federation (RC, 2006) [16]. Only two patients had been previously diagnosed with AIDS-defining conditions (stage 4B). Twenty patients (74%) had received ART, in various combinations, at the time of analysis (Table S1). Depending on HIV ribonucleic acid (RNA) concentration, the main group was divided into two subgroups. The first subgroup included 18 patients (67%) with undetectable HIV viral load (≤40 copies/mL). In nine patients (33%) in the second subgroup, HIV RNA concentration levels exceeded the detection threshold of the test system, and the median value was 3.9x105 copies/mL (range 6.7x104-1.1x106). Among the HIV-infected patients examined, HCV coinfection was detected in six patients (22%), and hepatitis B virus (HBV) coinfection was detected in three patients (11%). Therefore, 11 HCV-infected patients and 16 HBV-infected patients were examined as comparison groups (Table 1). All patients were treated at the Botkin Clinical Hospital for Infectious Diseases, which serves as a clinical trial setting for the Department of Infectious Diseases of Adults and Epidemiology (St. Petersburg State Pediatric Medical University). Diagnoses of chronic viral hepatitis C infection were confirmed by detection of HCV antibodies (anti-HCV) in peripheral blood and of HCV RNA. Diagnoses of chronic viral hepatitis B infection were confirmed by detection of HBV serological markers (HBsAg, anti-HBs IgG, anti-HBСor IgG, HBeAg, anti-HBe IgG) in patient peripheral blood and by the presence of HBV DNA.
| Group |
Number of Individuals |
Gender (Male/Female) |
Age (Years) Mean±SEM |
СD4+ cells/μL (Min-Max) |
|---|---|---|---|---|
| HIV-infected | 27 | 13/14 (48%/52%) |
37±7.5 | 465.5 (43-1669) |
| HCV-infected | 11 | 6/5 (54%/46%) |
49±12 | 706 (510-1268) |
| HBV-infected | 16 | 6/10 (37.5%/62.5%) |
53±12 | 571 (270-1213) |
| Healthy donors | 13 | 7/6 (54%/46%) |
38.5 ±15 | 962 (562-1611) |
Thirteen conditionally healthy donors, comparable in gender and age to the study group, were examined as the control group.
The research was carried out at the Laboratory of Molecular Immunology, Saint Petersburg Pasteur Institute. The study protocol was approved by the Ethics Committee of the Saint Petersburg Pasteur Institute in accordance with the Declaration of Helsinki. Written informed consent was obtained from each study participant.
The research material was blood, obtained by peripheral venipuncture, collected into vacutainers with K2EDTA. Analyses were performed within 24 hours of blood collection.
2.2. Flow Cytometry
The following combination of fluorochrome-conjugated anti-human monoclonal antibodies was used: CD32a/CCR4/CD3/CCR6/CXCR3/CD4. Staining was performed according to the standard protocols. In brief, 100 μl samples of whole peripheral blood were stained with the aforementioned antibodies in the dark at room temperature for 15 min. Erythrocytes were then destroyed by the addition of 1 ml of 1x BD Pharm Lyse (BD Biosciences #555899) and incubation for 15 min in darkness at room temperature. Next, samples were washed once with CellWASH (BD Biosciences #349524), spun for 7 min at 330g, and resuspended in 500 μl of 1x BD CellFIX (BD Biosciences #340181). Finally, data were acquired in the Novocyte flow cytometer (ACEA Biosciences, USA) and analyzed in NovoExpress (ACEA Biosciences, USA).
To identify CD32a+ Th cells, the following antibodies were used: CD32a-biotin (LifeSpan #LS-C84985-200, RRID: AB_1664735); CD3-PC5.5 (Beckman Coulter #A66327); and CD4-APC/Cy7 (BioLegend #357416, RRID: AB_2616810). The detailed flow cytometry gating strategy for identification of CD32a+ Th cells is shown in Fig. (S1). Briefly, from the total lymphocyte population, the total CD3+ T cell subset was identified. T cells were further analyzed for expression of CD4 (for identification of CD4+ Th cells). Th cells were finally gated for CD32a+ lymphocytes. To separate Th subpopulations, the following chemokine receptor antibodies were used: CCR4-PE (BioLegend #359412, RRID: AB_2562433); CCR6-PC7 (BioLegend #353418, RRID: AB_10916518); and CXCR3-APC (BioLegend #353708, RRID: AB_10983064). Th1 subpopulations had the CD3+CD4+CXCR3+CCR4-CCR6- phenotype. Th2 subpopulations had the CD3+CD4+CXCR3-CCR4+CCR6- phenotype. Th17 subpopulations featured the CD3+CD4+CXCR3-CCR4-CCR6+ phenotype [17].
All experiments, instrument setup, and quality control were performed in accordance with Guidelines for the Use of Flow Cytometry and Cell Sorting in Immunological Studies (second edition) [18].
2.3. Statistical Analysis
Continuous variables were compared between groups using non-parametric tests. Where three groups were compared, a Kruskal–Wallis test was used; pairwise comparisons were performed on all combinations of groups only if the overall test p-value was <0.05. Correlative analyses were performed using Spearman’s rank correlation.
3. RESULTS
3.1. CD32a Expression on T Helper Cells
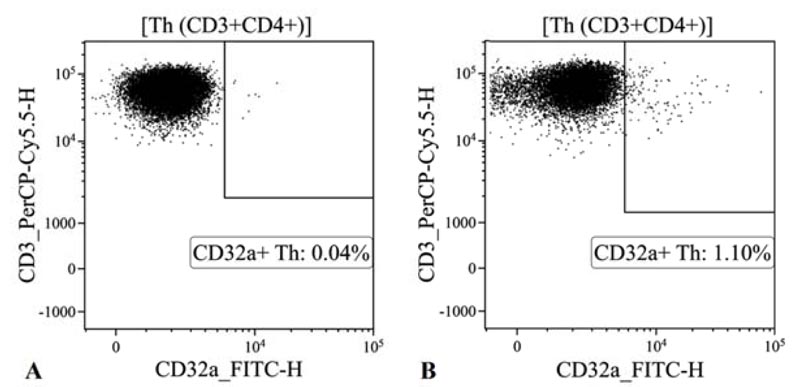
The research showed that the CD32a receptor expression on the surface of T helper cells (CD45+CD3+CD4+) is practically undetectable in conditionally healthy donors, in contrast to HIV-infected individuals (Fig. 1).
Fig. (2) shows the quantitation of CD32a expression on T helper cells from HIV-infected individuals or comparison groups. The relative concentration of CD32a+ receptor-expressing CD4+ T cells in the blood of HIV-infected patients was 14-fold higher than that in the blood of conditionally healthy individuals. It was also noticeably higher than that in the blood of patients infected with HCV (p=0.0008) or HBV (p˂0.0001). These results demonstrate that HIV infection leads to an increase in the relative concentration of CD32a+ T helper cells, while HCV/HBV infection does not affect the expression of the CD32a receptor on T helper cells.
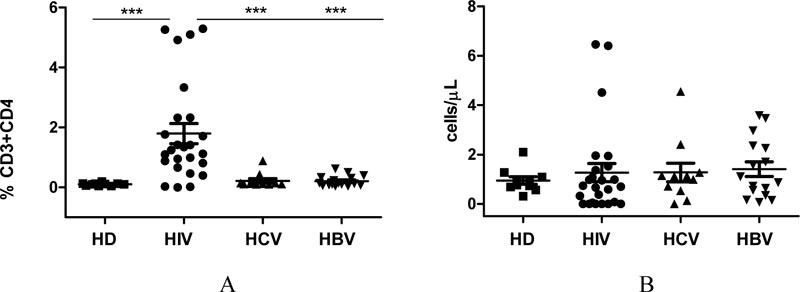
We found no differences in absolute CD32a+CD4+ T cell count in the examined groups. This can be explained by the fact that the absolute concentration of T helper cells is noticeably reduced in HIV-infected individuals, compared with: conditionally healthy individuals (p=0.0028); HCV-infected (p=0.029); or HBV-infected (p=0.037).
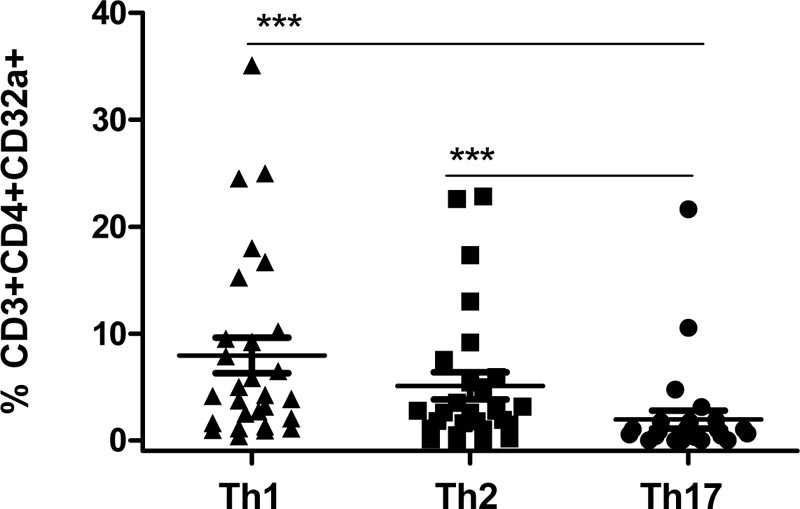
CD32a receptor expression in HIV-infected individuals was then assessed (Fig. 3) in relation to specific T helper cell subpopulations: Th1 (CD3+CD4+CXCR3+CCR4-CCR6-); Th2 (CD3+CD4+CXCR3-CCR4+CCR6-); and Th17 (CD3+CD4+CX CR3-CCR4-CCR6+). It was found that among T helper cells, the CD32a receptor was predominantly expressed on the surfaces of Th1 cells (median 4.18%, range 1.72%-10.02%, p<0.0001) and Th2 cells (median 2.61%, range 1.11%-5.92%, p<0.0001), as opposed to Th17.
3.2. Viral Load and CD32a Expression
Fig. (4) shows the relationship between CD32a receptor expression (on CD4+ T cells in HIV-infected individuals) and viral load values. A strong, direct correlation (r=0.78; p<0.0001) was found between viral load and CD32a+CD4+ T cell count in peripheral blood of HIV-infected individuals. Thus, the relative concentration of CD32a+ T helper cells in peripheral blood of HIV-infected individuals is associated with viral load level.

4. DISCUSSION
This research analyzed the expression of CD32a receptor on T helper cells in peripheral blood of HIV-infected patients. It was shown that CD32a expression was greatly increased on CD4+ T cells of HIV-infected individuals, compared with that in conditionally healthy donors, HCV-infected patients, or HBV-infected patients. Thus, HIV infection affects CD32a expression on the surface of T helper cells. Earlier, Badia R. et al. (2018) reported significantly higher CD32 expression in CD4+ T cells from HIV-1 infected individuals, when compared to uninfected controls [13]. It is known that after activation, naive CD4+ T cells express FcγRIIIa. After binding to an immune complex, this expression provides a costimulatory signal necessary for the differentiation of these cells into a population of effector cells. This is supported by studies that have discovered predominant expression of CD32a on the central memory T cell (Tcm cell) subpopulation in HIV-positive and healthy individuals [19].
Whole peripheral blood, collected from HIV-infected individuals and healthy donors, served as our study material. T cells were phenotyped within 24 hours of blood collection, so that lymphocytes were not subjected to isolation of PBMCs using density gradients, freezing, or thawing. Previous research has shown that the concentration of CD32+CD4+ cells is greatly reduced in frozen PBMCs, compared with that in fresh PBMCs [12]. Moreover, the isolation of CD32+CD4+ T cells by negative selection can lead to a severe loss of cells [10]. We used antibodies capable of distinguishing CD32a from CD32b in order to unambiguously identify CD32a+CD4+ T cells. We also used chemokine receptor antibodies in order to identify T helper subpopulations (Th1, Th2, Th17) and to determine which T helper subpopulations CD32a receptor is predominantly expressed.
Approximately 8-16% of HIV-infected individuals have HCV and/or HBV coinfection [20]. Despite the fact that existing literature has no information on any connection between CD32a expression and viral hepatitis infection, patients with HCV or HBV monoinfection were examined as comparison groups in order to eliminate any impact these infections might have on CD32a expression analysis (on T helper cells of HIV-infected patients).
For the first time, it was found that the CD32a receptor is predominantly expressed on Th1 and Th2 cells. Previously, Abdel-Mohsen et al. (2018) reported that among memory T helper cells, the Th2 subpopulation predominantly expressed CD32 [12]. This disparity can be explained by the fact that in the study by Abdel-Mohsen, PBMCs served as the study material, while the authors were determining a pan-CD32 antigen on T helper subpopulations. In contrast, whole peripheral blood served as our study material. We used monoclonal antibodies that allowed us to isolate the CD32a receptor isoform, and our results were obtained from a general CD4+ T cell subpopulation, without dividing it into groups according to cell differentiation stages.
We have shown that CD32a is expressed mainly on the Th1 subpopulation (CD3+CD4+CXCR3+CCR4-CCR6-). Previous studies have shown that CD4+ cells expressing the CXCR3 chemokine receptor are the major source of infectious HIV-1 in blood [21]. It was established that CXCR3+CCR6+ T helper cells in peripheral blood contain substantial quantities of viral RNA [22]. Moreover, it was shown that proliferation of HIV-infected Th1 cells plays a key role in HIV persistence [23]. Thus, the CD4+CXCR3+CD32a+ subpopulation of Th cells may be of further interest in understanding the contribution of these cells to HIV persistence.
We found a strong, direct correlation between viral load and CD4+ T cell count expressing CD32a in peripheral blood. Wittner et al. (2018) showed that in patients with a high viral load, CD32 expression is noticeably higher on memory T cells, but not on naive T cells [24]. Our data (showing the relationship between viral load and CD32a+CD4+ cell counts) do not support the assertion that CD32a is a marker of latent HIV reservoirs. Rather, on the contrary, they indirectly confirm the results obtained by Abdel-Mohsen et al. (2018), demonstrating that with infected cells both in vitro and in vivo, CD32 is mainly expressed in activated CD4+ T cells carrying HIV RNA [12].
CONCLUSION
In summary, it should be stated that our research was performed on clinical samples. The study material was peripheral blood that was not exposed to isolation of PBMCs, freezing, or isolation of cells by magnetic separation. A CD32a mouse monoclonal antibody was used to identify human CD32a+ cells. With a high level of certainty, we found that CD32a receptor expression on T helper cells of HIV-infected individuals was 14-fold higher compared with that of conditionally healthy donors. The levels of CD32a expression on CD4+ T cells in patients infected with HCV or HBV did not differ from levels in healthy donors. We have shown that relative CD32a+ T helper cell count in peripheral blood of HIV-infected individuals has a strong, direct correlation with viral load level. For the first time, it was found that the CD32a receptor is predominantly expressed on Th1 and Th2 cells. In conclusion, the CD32a receptor can be considered a marker of infection, and CD32a+CD4+ T cell count directly correlates with viral load level in HIV-infected individuals.
AUTHORS' CONTRIBUTIONS
All authors contributed to the study’s conception and design. The supervisor of this study is Areg A. Totolian. Examination of patients, diagnoses, and material preparation was performed by Elena V. Esaulenko, Ekaterina V. Boeva, and Alexey Y. Kovelenov. Methodology: Igor V. Kudryavtsev. Data collection and analysis were performed by Natalia A. Arsentieva and Oleg K. Batsunov. The first draft of the manuscript was written by Natalia A. Arsentieva; all authors commented on previous versions of the manuscript. Funding acquisition and writing (review, editing): Areg A. Totolian and Alexander V. Semenov. All authors have read and approved the final manuscript.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the Ethics Committee of the Saint Petersburg Pasteur Institute, Russia (No. 11, dated 17/11/2019).
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
Written informed consent for publication was obtained from each study participant.
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of the article are available at the Laboratory of Molecular Immunology, St. Petersburg Pasteur Institute. Data access can be provided upon request.
FUNDING
This study was supported by the Saint Petersburg Pasteur Institute within the framework of a State Task (Registration No АААА-А16-116061410018-9).
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
We thank the participants enrolled in this study in addition to the participating organizations.
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publisher’s website along with the published article.


