All published articles of this journal are available on ScienceDirect.
The Impact of Universal Test and Treat Program on Highly Active Anti Retroviral Therapy Outcomes (Coverage, Adherence and Lost to Follow Up) at Wangaya Hospital in Denpasar, Bali-Indonesia: A Retrospective Cohort Study
Abstract
Background:
World Health Organization (WHO) (2015) recommended that all people diagnosed with human immunodeficiency virus (HIV)-positive initiate Highly Active Anti Retroviral Therapy (HAART) immediately (less than a week), irrespective of CD4 count (Universal Test and Treat / UTT) Program.
Objective:
To evaluate the impact of UTT as a current therapeutic program on HIV treatment outcomes, coverage, adherence, and lost to follow-up (LTFU) at Wangaya Hospital in Denpasar, Bali, Indonesia.
Methods:
A Retrospective cohort study was conducted during July 2017 - June 2018 (Pre-UTT) and September 2018 – August 2019 (Post-UTT). Around 402 medical records were selected, reviewed, and enrolled. Data were analyzed using SPSS software for windows version 24.0. Bivariate analysis (Chi-square test) was performed on all variables with a statistically significant t level of 0.05.
Results:
Among 4,322 new visitors; 3,585 (82.95%) agreed to take HIV test and 402(11.21%) were confirmed HIV reactive. Most participants confirmed HIV reactive occured at age 25-34 years old and 230 (57.21%) were male. The majority education level were primary (Junior high school) 302(75.12%), 379(94.28%) were employed and 281 (69.90%) stayed in Denpasar. About 350 (87.06%) received HAART, 298 (85.14%) with high adherence and 52 (14.86%) LTFU. Pre-UTT, HAART coverage; 83.03% (181), were statistically significant increased to 91.85% (169) post UTT (p=0.000). High adherence pre-UTT; 79.56% (144) was significantly increased to 91.12% (154) post UTT (p=0.006) and LTFU were significantly decreased; 20.44% (37) to 8.87% (15) (p=0.006).
Conclusion:
UTT program significantly improve the HIV treatment outcome (increased coverage, adherence, and decreased LTFU).
1. INTRODUCTION
With over 35 million deaths reported, the Human Immunodeficiency Virus (HIV) remains one of the major public health threats; there were still over two million infections in 2016 [1,2]. Indonesia is one of the HIV hard-hit countries with HIV prevalence among adults between the ages of 15 and 49 years old reached 0,3% in 2015. WHO recommended HIV-positive people to initiate HAART, irrespective of CD4 cell count, by implementing Universal Test and Treat (UTT) program [3-7]. The Joint United Nations Program on HIV/AIDS (UNAIDS) has a worldwide target; by 2020, at least 90% of people will be diagnosed with HIV infection, at least 90% of those will receive HAART, and at least 90% of those receiving HAART will have suppressed viral replication (The 90-90-90 target). Full achievement of 90–90–90 is equal to viral load suppression among 73% of all people living with HIV. [3,7-10]. Indonesia's success in raising the number of people screened for HIV has been considerable. In addition, the number of people on antiretroviral therapy (ART) increased from just a few thousand in early 2011 to over 73,000 in September 2016. In 2016, only 32.1% of (69,954 of the 217,631) HIV patients were receiving ARV therapy. However, this coverage is inadequate to accomplish the 2020 objectives [11]. Based on the findings of the joint Indonesian and International Review Team, the Ministry of Health should consider expanding eligibility for ART to all people living with HIV, regardless of CD4 count [12]. Universal Test and Treat (UTT) is a current HIV therapy program that gives HAART as soon as possible, irrespective of CD4 cell count. The people at risk are screened for HIV infection, whereas people who are diagnosed with HIV infection are given early HAART. The HIV outcome improvement (increased the coverage, adherence and decreased the lost to follow up, viral suppression, reduce HIV transmission to other people, and finally increased quality of life) is the target of UTT [13-17]. Since September 2018, Wangaya Hospital in Denpasar, Bali, Indonesia, has implemented the UTT program.
2. METHODS
2.1. Settings and Design
A retrospective cohort study was conducted during July 2017- June 2018 (Pre-UTT) and September 2018 - August 2019 (Post-UTT) at Merpati Clinic at Wangaya Hospital in Denpasar, Bali, Indonesia, which included a total of 402 participants.
2.2. Materials and Methods
A retrospective cohort study of HIV therapy outcomes (coverage, adherence, and lost to follow-up) was conducted at Wangaya Hospital in Denpasar, Bali, Indonesia. The cohorts with equal follow-up duration: Pre-UTT and Post-UTT. In the pre Test and Treat, participants who started HAART in July 2017 were enrolled. The criteria for CD4 counts to start HAART (Pre-UTT) was ≤ 350 cell/L, and on Post-UTT, HIV therapy program that gives HAART as soon as possible, irrespective of CD4 cell count.. The post Test and Treat included participants who started HAART in September 2018.
2.3. Data Collection and Definition of Outcomes
All data were obtained from regularly collected medical record paper. Four data collectors and two supervisors who were health practitioners and employees at ART clinics were hired for data collection after receiving training on the method.
We used standardized The Indonesia Ministry of Health (MOH) HAART outcomes. Lost to follow-Up (LTFU) is the participants who did not return to Health care (Wangaya Hospital) for six months or they returned to the clinic after their six months absence and were not known to have referred out, died or stopped HAART. Participants who were referred to care to other HIV clinics were classified as Transfer out.
2.4. Adherence Measurement
The types of ARV given to the participants were fixed dose combination (Tenofovir, Lamivudin and Efavirenz) and non fixed dose combination (Tenofovir, Lamivudin, Nevirapine / Zidovudine, Lamivudin, Efavirenz / Zidovudine, Lamivudin, Nevirapine).
In this study, the pill counts were used to measure the HAART adherence by counting the rested pills numbers in the participants’ bottles. The participants carried back the containers of pills to the clinicians to count the leftover pills. Pill counts were assessed retrospectively based on 30 days recall. The index of adherence was calculated by the formula:
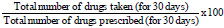 |
The high adherence was considered for the participants with ≥95% of adherence, and low adherence was less than 95% of adherence [14-19].
2.5. Data Analysis
The researcher analyzed the descriptive statistics of all the HIV-positive patients that are on ART treatment at the health center. The demographic analysis included: age, sex, level of education, employment status, address, HIV test, HIV reactive, taking ARV, type of ARV, adherence, and lost to follow up.
Later, a bivariate analysis was run for the significant variables to determine the significant factors that may affect the universal test and treat strategy on HIV treatment outcomes (coverage, adherence, and lost to follow up).
Data were compared between the Pre UTT with the Post-UTT by using SPSS software for windows version 24.0. Chi-square test was done with a statistically significant level of 0.05.
3. RESULTS
Among 4,322 new visitors (2,259 pre-UTT and 2,063 post-UTT), 3,585 (1,852 pre-UTT and 1,733 post-UTT) (82.95%) agreed to take HIV test and 402 (218 pre-UTT and 184 post-UTT) (11.21%) were confirmed HIV reactive. Most participants with confirmed HIV reactive were 25-34 years old, and 230 (57.21%) were male. The majority education level was primary (Junior high school) 302(75.12%); 379(94.28%) were employed, and 281 (69.90%) stay in Denpasar (Table 1).
The number of participants who received HAART (coverage) immediately after HIV diagnostic confirmation was 350 (87.06%), with the proportion was 181 (83.03%) pre-UTT and 169 (91.85%) post-UTT. The types of ARV were fixed-dose combination (FDC) and non-FDC with predominance taking FDC 148 (81.77%) Pre-UTT increases to 158 (93.50%) Post-UTT.
The participants with high adherence were 144 (79.56%) pre-UTT and 154 (91.12%) post-UTT. The lost to follow up were 37 (20.44%) pre-UTT and 15 (8.87%) post UTT (Table 1).

3.1. Pre–UTT
Among 2259 new visitor participants pre-UTT, 1852 (81.97%) visitors who took HIV test, 218 (11.77%) were confirmed with HIV positive, and 181 (83.03%) accepted ART. After 2 months follow up, there were 37 (20.44%) participants LTFU with the reasons; 12(34.04%) feeling healthy, 13 (36.17%) due to adverse effect of ART, 2 (4.25%) busy, 6 (17.02%) far from home and 4 (8.51%) participants were dead (Fig. 1).
3.2. Post-UTT
Among 2,063 new visitor participants post-UTT, 1,733 (84.00%) visitors who took HIV test, 184 (10.62%) were confirmed with HIV positive, and 169 (91.85%) accepted ART. After 2 months follow up, there were 15 (8.87%) participants LTFU with the reasons; 5(36.66%) feeling healthy, 7 (46.66%) due to adverse effect of ART, 0 (0%) busy, 1 (6.66%) far from home and 2 (10.00%) participants were dead (Fig. 1).
4. DISCUSSION
UTT is an intervention strategy that allows the screening of the HIV high-risk population and those who are diagnosed HIV infection to extend the initiation treatment immediately, aiming to eliminate HIV as it reduces the spreading rate of the virus to other people [15, 18-22].
UTT program has improved the participants’ outcome; it leads the participants to find medical support early. Meanwhile, the fatal of opportunistic infections might be prevented by early treatment and prophylaxis [23-26].
This study compared the HIV treatment outcome 1-year pre-UTT with post-UTT. The impact of the Universal Test and Treat Program on HIV treatment outcomes; coverage, adherence and lost to follow up (Table 2).
4.1. Comparison Treatment Coverage Between Pre-UTT and Post-UTT
This study found that treatment coverage; 181 (83.03%) pre-UTT and a statistically significant increased of ARV to 169 (91.85%) in post-UTT (p = 0.000). Other study reported, from 3,256 people living with HIV/AIDS (PLWHA) included in their study 2,652 (81.00%) were Test and Treat patients [27]. Onoya D (2020) found that an early ART rates elevated with implementation time of longer policy (aRR 0.2 for < 3 months vs > 10 months, 95% CI 0.1 to 0.4) [3]. Mc Manus H (2019) found the proportion of patients who given early treatment elevated from 17% (15 patients) in 2004-2006 to 20% (34 patients) in 2007-2009, 34% (95 patients) in 2010-2012 and 53% (197 patients) in 2013-2015 (trend, p<0.001) [28].
| - |
Total (N=402) |
Pre-UTT (N=218) |
Post-UTT (N=184) |
|---|---|---|---|
| Gender | - | - | - |
| Male | 230 (57.21%) | 117 (53.67%) | 113 (61.41%) |
| Female | 172 (42.79%) | 101 (46.33%) | 71(38.59%) |
| Age (Year) | - | - | - |
| 18-24 | 45(11.19%) | 16 (7.34%) | 29 (15.76%) |
| 25-34 | 142(35.32%) | 78 (35.78%) | 64 (34.78%) |
| 35-44 | 127(31.59%) | 79 (36.24%) | 48 (26.09%) |
| >44 | 88(21.89%) | 45 (20.64%) | 43(23.37%) |
| Education | - | - | - |
| Less than primary (Elementary school) | 41(10.20%) | 21 (9.63%) | 20(10.87%) |
| Primary (Junior high school) | 302(75.12%) | 158 (72.48%) | 144(78.26%) |
| Secondary or higher (University) | 59(14.68%) | 39 (17.89%) | 20(10.87%) |
| Employment status / occupation | - | - | - |
| Employed | 379(94.28%) | 207 (94.95%) | 172 (93.48%) |
| Unemployed | 23(5.72%) | 11 (5.05%) | 12 (6.52%) |
| Address (travel burdens) | - | - | - |
| Denpasar | 281(69.90%) | 162 (74.31%) | 119 (64.67%) |
| Out of Denpasar New visitors |
121(30.10%) 4322 |
56 (25.69%) 2259 |
65 (35.33%) 2063 |
| HIV test | 3585(82.95%) | 1852 (81.98%) | 1733 (84.00%) |
| HIV reactive | 402(11.21%) | 218 (11.77%) | 184 (10.62%) |
| Taking ARV (HAART) | 350(87.06%) | 181 (83.03%) | 169 (91.85%) |
| Type of ARV (due to adverse effect) | - | - | - |
| FDC | 306(87.43%) | 148 (81.77%) | 158 (93.50%) |
| Non-FDC Adherence High adherence Low adherence |
44(12.57%) 298 (85.14%) 52 (14.86%) |
33 (18.23%) 144 (79.56%) 37 (20.44%) |
11 (6.50%) 154(91.12%) 15 (8.87%) |
| Lost to Follow up | 52 (14.86%) | 37 (20.44%) | 15 (8.87%) |
UTT: Universal Testing and Treatment, FDC: Fixed Dose Combination
| - | Total (N=402) |
Pre-UTT (N=218) |
Post-UTT (N=184) |
P value |
|---|---|---|---|---|
| Gender | - | - | - | - |
| Male | 230 (57.21%) | 117 (53.67%) | 113 (61.41%) | 0. 072 |
| Female | 172 (42.79%) | 101 (46.33%) | 71(38.59%) | - |
| Age (Year) | - | - | - | - |
| 18-24 | 45(11.19%) | 16 (7.34%) | 29 (15.76%) | 0.053 |
| 25-34 | 142(35.32%) | 78 (35.78%) | 64 (34.78%) | - |
| 35-44 | 127(31.59%) | 79 (36.24%) | 48 (26.09%) | - |
| >44 | 88(21.89%) | 45 (20.64%) | 43(23.37%) | - |
| Education | - | - | - | - |
| -Less than primary (Elementary school) | 41(10.20%) | 21 (9.63%) | 20(10.87%) | 0.139 |
| -Primary (Junior high school) |
302(75.12%) | 158 (72.48%) | 144(78.26%) | - |
| -Secondary or higher (University) |
59(14.68%) | 39 (17.89%) | 20(10.87%) | - |
| Employment status / Occupation | - | - | - | - |
| Employed | 379(94.28%) | 207 (94.95%) | 172 (93.48%) | 0.336 |
| Unemployed | 23(5.72%) | 11 (5.05%) | 12 (6.52%) | - |
| Address (travel burdens) | - | - | - | - |
| Denpasar | 281(69.90%) | 162 (74.31%) | 119 (64.67%) | 0.058 |
| Out of Denpasar New visitors |
121(30.10%) 4322 |
56 (25.69%) 2259 |
65 (35.33%) 2063 |
- |
| HIV test | 3585(82.95%) | 1852 (81.98%) | 1733 (84.00%) | - |
| HIV reactive | 402(11.21%) | 218 (11.77%) | 184 (10.62%) | - |
| Taking ARV (HAART) | 350(87.06%) | 181 (83.03%) | 169 (91.85%) | 0.000* |
| Adherence High adherence Low adherence |
298 (85.14%) 52 (14.86%) |
144 (79.56%) 37 (20.44%) |
154(91.12%) 15 (8.87%) |
0.006* |
| Lost to Follow up | 52(14.86%) | 37 (20.44%) | 15 (8.87%) | 0.006* |
UTT: Universal Testing and Treatment, FDC: Fixed Dose Combination
A Rufu et al. (2018) demonstrated that among 972 participants diagnosed with HIV, 915 (94.00%) enrolled for HIV care and 771 (79.00%) given ART [24]. Hayes R, 2017; the proportion estimation of known HIV-positive individuals on ART elevated overall from 54.0% after the community HIV-care providers (CHiP) visit to 74.0% by the end of the round for men and from 53.0% to 73.0% for women [29]. Petersen M et al (2017) found participants intervention was 80.2% (95% CI, 79.1%-81.2%) and an elevation after 2 years 93.4% had given ART (95% CI, 92.8%-94.0%) [4]. Luo S et al. (2015) reported that the ART coverage for PLWHA increased to 58% since 2013 [30].
4.2. Comparison Adherence Between Pre-UTT and Post-UTT
We found that the participants with high adherence, pre-UTT 144 (79.56%), increased significantly to 154 (91.12%) in post-UTT (p=0.006). Another studies found that in 12 months retention on ART in the UTT cohort was higher than in the pre-UTT cohort 83.0% (95% CI: 81.0% to 85.0%) vs 76.2% (95% CI 73.9% to 78.5%) [7].
4.3. Comparison Lost to Follow Up Between Pre-UTT and Post-UTT
This study found that the LTFU pre-UTT 37 (20.44%) decreased to 15 (8.87%) (p=0.006). In another study, Alhaj M et al. (2019) found that the LTFU decreased 16.4% (82) pre-UTT to 13.5% (134) in post-UTT [7]. Stafford KA (2019) had demonstrated that in 12 months post-ART initiation, 34.00% of the participants who initiated ART under the UTT strategy were LTFU [27].
Our research provides HIV service delivery partners with essential information to assist in improving the health system and accelerate the development of treatment nationally. Our study agrees with other studies in showing that a house-to-house service and expanding access to ARV services through health centers can achieve high rates of testing and knowledge of HIV status among community members. Based on our findings, at the facility level, national expectations related to therapy and follow-up, including regular interaction following initiation of treatment and identification of barriers to care, may be considered.
CONCLUSION
In this study, we found the impact of the Universal Test and Treat program on HIV treatment outcomes, coverage and adherence were significantly increased, and Lost to Follow Up were decreased.
THE LIMITATION OF THE STUDY
Readability of the recorded data from medical record paper and incompleteness of information remains major attention since the study was started.
AUTHOR'S CONTRIBUTIONS
Authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. Other staff contributed to the data collection.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The procedure of the study was approved by Wangaya Hospital Denpasar, Bali, Indonesia Ethical Committee with register number: 03/RSUDW/Litbang/2017).
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All human research procedures were followed in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
A written consent form was provided to the participants wherein the procedure of the study, its risks and benefits, confidentiality was explained to the participants. The participants were also made aware that they are entitled to the right of withdrawal from the study at any time. Only participants who gave their consent by signing the consent form were recruited into the study.
AVAILABILITY OF DATA AND MATERIALS.
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGMENTS
We would like to thank the Director of Wangaya Hospital, all of the participants and the family, Wangaya HIV Study Group staff, all of our colleagues who supported this study, and Mrs. Puji Astuti et al., who contributed to the data collection.


