All published articles of this journal are available on ScienceDirect.
Male Circumcision and HIV Transmission; What Do We Know?
Abstract
Male circumcision (MC) has been shown to be protective against heterosexual HIV transmission and is being explored in some parts of the world as a means of combating the epidemic. The World Health Organization (WHO) recommends that MC be considered as an important component of HIV prevention in high prevalence settings.
We review evidence that demonstrates that the inner foreskin is likely to be the main portal of entry for the HIV virus in males. Whether removal of the inner foreskin accounts for all the protection afforded by circumcision is yet to be established.
The proposed mechanisms of protection range from inherent immunohistological factors of foreskin such as difference in thickness of keratin layer and density of target cells for HIV between inner and outer foreskin to physiological mechanisms that follow male circumcision such as drying of secretions underneath foreskin after sexual intercourse, loss of microbiome that attract target cells to the genital mucosa and lack of priming the genital mucosa with less abundant sexual transmitted infections among circumcised men.
The aim of this review is to give an updated account on the mechanisms proposed so far on the demonstrated 50-70% protection from HIV transmission through heterosexual intercourse, by male circumcision.
INTRODUCTION
The United Nations Programme on HIV/AIDS (UNAIDS) estimated that there were 35.3(32.2-38.8) million people living with HIV by the end of 2012 [1]. Since peaking in 1999, the annual number of new HIV infections has been in steady decline. However, due to antiretroviral therapy and subsequent reduction in AIDS-related deaths HIV is still increasing in prevalence [1]. This means HIV remains a significant public health issue on a global scale.
Three landmark phase III clinical trials of male circumcision (MC) conducted in South Africa, Kenya and Uganda demonstrated that MC has an efficacy of around 60% in preventing heterosexual HIV acquisition in men [2-4]. These findings confirmed earlier observational and ecological studies and prompted the World Health Organization (WHO) recommendations that MC be considered an important component of a comprehensive HIV prevention packages where: HIV prevalence is high, most infections are transmitted through heterosexual sex, there are low prevalence of existing MC and where sexual health counselling can be conducted [5].
The three intervention studies that demonstrated a reduction in HIV acquisition in men who had been circumcised proposed a number of mechanisms to explaintheir findings. All three proposed the removal of inner foreskin (IFS) which has higher number of cellular receptors for HIV as a mechanism for reduced HIV transmission. Beyond the number of receptors, three groups proposed different mechanisms to explain their results. These included: increased keratin thickness of the glans penis when the protection from foreskin is absent, more rapid genital drying after intercourse in circumcised men, and reduction of the surface area of the penis after circumcision [2-4]. One study highlighted the heightened susceptibility of cellular receptors to HIV infection [3], while differential keratin thickness between inner and outer foreskins plus micro-trauma was highlighted in another study [4]. Given the varied mechanisms proposed for the protection afforded by MC, it is timely to review the current evidence of how MC protects an individual from HIV infection.
METHODS
We searched MEDLINE, Web of science, Scopus and Cochrane reviews electronic databases using ‘‘male circumcision” and “HIV transmission’’ (as paired key words) as both text words and medical subject heading (MeSH) terms. Only the articles published in English language during the period of 1981-2013 were searched. In addition, articles identified by citations in discovered articles were retrieved, and duplicate records were removed. The eligibility of articles was assessed based on titles and abstracts and the full texts of eligible articles were reviewed for content. Only original research was included in the systematic review of mechanisms. For the purpose of this review, anatomical and histological terms were standardised as per Fig. (1). The histological term “epithelium” which is used in some literature is referred to here as the outer foreskin (OFS) or epidermis according to context, while the term “mucosa” is referred to as the inner foreskin (IFS), and “submucosa” as dermis. Studies are collated under 8 main headings which cover the main areas of research into the biological mechanisms of HIV transmission across the foreskin and protection afforded by removing the foreskin.
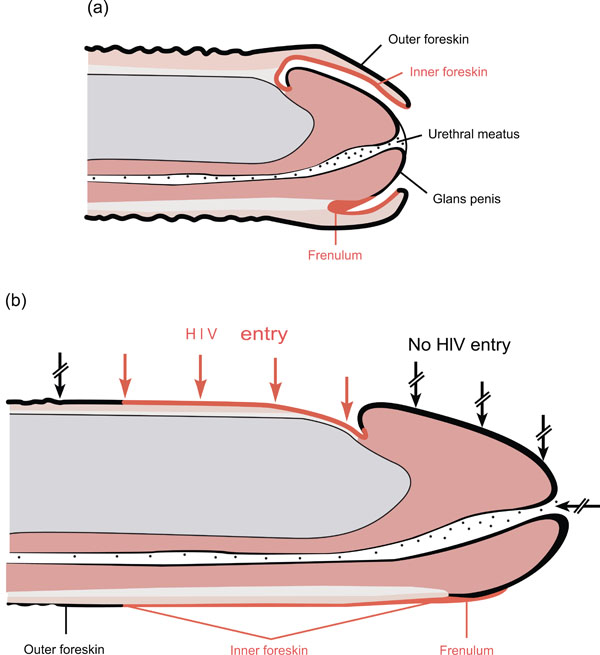
(a) Flaccid uncircumcised penis. (b) Erect uncircumcised penis with foreskin retracted showing likely sites of HIV-1 entry (Reproduced with permission) [6].
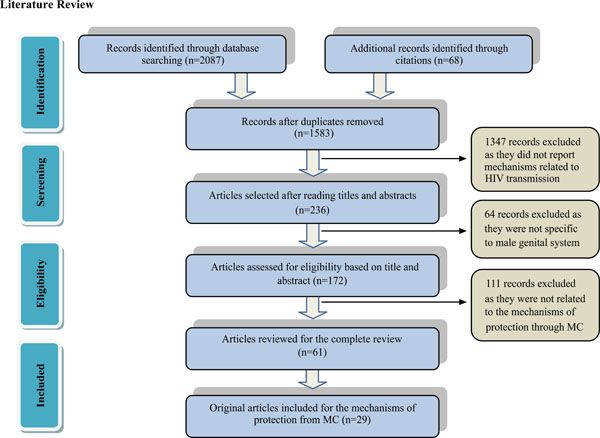
Prisma diagram illustrating the method of selecting articles.
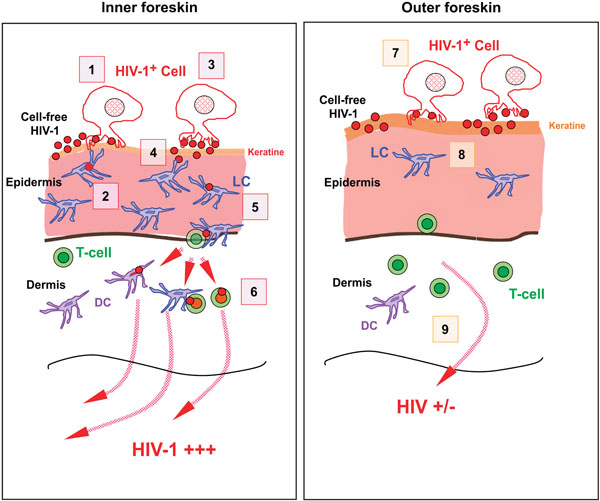
Schematic diagram showing the suggested initial events occurring in the foreskin during HIV (type-1) transmission. (Left) In inner foreskin, while cell-free HIV-1 and lightly HIV-1 infected cells unlikely to initiate and disseminate infection through inner foreskin, the cells infected heavily with virus easily infects the target LC cells in epidermis and disseminate infection at dermis through DCs and T cells (1-6). (Right) In outer foreskin, both newly budded HIV-1 particles and cell-free HIV-1 virions remain trapped within the thick layer of keratin at OFS limiting virus entry into the epidermis and infection being disseminated (7-9). (Reproduced with permission) [22].
MECHANISMS SUGGESTED FOR PROTECTION
Keratin Thickness Differences of Foreskin Epithelium (Table 1)
Several studies have been published on keratin layer thickness of inner and outer foreskins which is believed to be the first line defence for the entry of HIV into the penile tissues. McCoombe and Short’s (2006) study showed the IFS (mean thickness, 1.8 units; SE, 0.1) is significantly less keratinized than the OFS (mean thickness, 3.3 units; SE, 0.1) or glans penis (mean thickness, 3.3 units; SE, 0.2; P < 0.05) using cadaveric samples of HIV sero-negative individuals obtained within 18 hours of death [6]. In this study, keratin thickness was subjectively assessed on a scale of 0-5 arbitrary units, where 0 corresponded to no keratin, and 5 to maximum keratinization (keratin thickness ≥ 20 µm). There was no difference in keratinisation between the OFS and the glans penis. The frenulum and the urethral meatus were less keratinized, and there was no keratin observed in the penile urethra [6]. Similarly, in their publication, Patterson and colleagues reported that the extent of keratinisation was much greater in the OFS surface than the IFS surface though actual data on keratin thickness were not provided [7]. Ganor and Bomsel, in their later review, reported a higher number of apical keratin layers in OFS that make OFS thicker and denser compared to IFS at ultra-structural level [8].
In contrast, Dinh and colleagues found no clear difference in keratin thickness between the inner and outer foreskin in their study that included 16 consenting male participants undergoing circumcision for medical conditions, mainly phimosis [9]. Keratin of the IFS was thicker than that of the OFS in seven of their donors; the keratin of OFS was thicker than the IFS of three donors; and no significant difference was seen between the two aspects of six donor foreskins. Intra-individual variability in the measured keratin thicknesses of some donors was also noted. Furthermore, a significant heterogeneity between the donors was observed. The authors attributed that to genetic differences in skin composition and exposure to environmental stimuli over an individual’s lifespan that could induce changes in the expression of epidermal proteins such as filagrin or keratin [9]. Dinh and colleagues published a similar study in 2012 with foreskins from 19 healthy men who underwent prophylactic circumcision in Uganda [10]. This study was conducted to avoid the possible shortcomings of their previous study; i.e., using foreskin samples taken from men circumcised for foreskin pathologies and the probable inadequate representation of different sites of foreskin in the analysis. Yet, the results of this study were consistent with their previous results that showed no difference in thickness of keratin layer between inner and outer foreskins [10] (Table 3).
Summary of studies that examined the keratin thickness of the foreskin.
| STUDY | SUBJECTS | INDICATION FOR CIRCUMCISION | STI STATUS | SPECIMEN PROCESSING & MICROSCOPY | RESULTS & CONCLUSIONS |
|---|---|---|---|---|---|
| Patterson, 2002 [7] | 14 subjects10 months - 69years from Chicago, USA |
Phimosis, Balanitis,
Adhesions (3 subjects) Religious, Cultural or cosmetic (11subjects) |
Not specified | Processed within: 3 hours Fixed with: Streck Tissue Fixative Embedded in: Paraffin or OCT Section size: 5µm Stained with: immunohistochemistry Examined with: MetaMorph software V4.5 | IFS (data not shown) OFS (data not shown) Extent of keratinisation was much greater in the OFS surface than the IFS. Concluded that keratin layer of OFS is thicker than IFS. (OFS>IFS) |
| McCoombe, 2006 [6] |
9
cadavers† (mean age 77.4 years) 21 subjects† (mean age 28.9 years) 8 penile necropsy specimens† (mean age 30.9years) from Melbourne, Australia |
Not specified | HIV negative | Processed within: necropsy specimens obtained within 18 hours of death Fixed with: Formalin Embedded in: Paraffin Section size: 8µm Stained with: H & E stains and Ayoub-Shklar stains Examined with: Light microscopy 200-400 magnification | IFS - 1.8 units OFS - 3.3 units (Units; 0-no keratin 5- max keratin> 20 µm assessed subjectively) Concluded that IFS and frenulum are protected by a much thinner layer of keratin than those in the glans penis or OFS (OFS>IFS). |
| Qi Qin, 2009 [11] |
80
subjects, 60
were 2-7 years 20 were 20-29 years from Hangzhou, China |
Phimosis or redundant foreskin with UTI (30 boys) Cultural or cosmetic (30 boys) Not specified for adults who were otherwise healthy | Not specified | Processed within: Not specified Fixed with: 4% Para formaldehyde Embedded in: Paraffin Section size: 4µm Stained with: H & E Examined with: Light microscopy 100-400 magnification | IFS - 12.1 ± 4.1 µm OFS - 9.3 ± 2.0 µm Concluded that OFS is less keratinized than the IFS in healthy adults and in boys with infectious history (IFS > OFS). |
| Dinh, 2010 [9] | 16 subjects† from Chicago, USA | Phimosis | Not specified | Processed within: Not specified Fixed with: 3.7% Formaldehyde, 0.1mol/l PIPES buffer Embedded in: Paraffin, OCT Section size: 10µm ( cryosections) Stained with: immunofluorescence and H & E Examined with: DeltaVision RT systems using Softworx software |
IFS
(data not shown) OFS (data not shown)
Keratin thickness of 7 subjects- IFS > OFS Keratin thickness of 3 subjects- IFS < OFS Keratin thickness of 6 subjects- No difference Concluded no difference between the IFS and OFS keratin layers (IFS=OFS). |
| Dinh, 2012 [10] | 19 subjects 21-41 years from Rakai, Uganda | As prophylactic measure for HIV acquisition | HIV negative No evidence of current STIs | Processed within: Not specified Fixed with: 3.7% Formaldehyde, 0.1mol/l PIPES buffer Embedded in: OCT Section size: 10 µm Stained with: immunofluorescence and H & E Examined with: DeltaVision RT systems using Softworx software | IFS - 14.676 ± 7.48 µm OFS -13.306 ± 8.49 µm frenar band -16.916 ± 12.42 µm Concluded no difference between the IFS and OFS keratin layers (IFS=OFS). |
† Age range not specified.
(IFS, inner foreskin; OFS, outer foreskin; LC, Langerhans cells STI, sexually transmitted infection PBS, phosphate buffered solution OCT, Optimum Cutting Temperature).
Studies that examined target cell distribution in foreskin tissues.
| STUDY | SUBJECTS | INDICATION FOR CIRCUMCISION | STI STATUS | RESULTS (LCs, CD4 T cells & Macrophages) & CONCLUSIONS |
|---|---|---|---|---|
| Hussain, 1995 [19] |
10 subjects, 7 subjects, 3-7 weeks 3 subjects, 7-36 years from London, UK |
Not Specified | Not specified | LCs: Infants: 41.9 ± 4.6 cells/mm2 in IFS, density in OFS not enumerated. Older: 26.0 ± 5.7 cells/mm2 in IFS, density in OFS not enumerated. No significant difference between IFS versus OFS terms of LC density (IFS = OFS).CD4+T cells: Large number of CD4+T cells found in dermis and small number was found in epidermis. Macrophages: Found in dermis |
| Patterson, 2002 [7] | 14 subjects, 10 months- 69 years from Chicago, USA | Phimosis, balanitis, adhesions and redundant foreskins. | Not specified | LCs : Significantly greater in IFS compared to OFSγ (IFS > OFS) Majority of LCs found in epidermis (inner and outer).CD4+ T cells: Significantly greater in IFS compared to OFSγ (IFS > OFS) Majority of CD4 T cells found in dermis.Macrophages: percentage* of macrophages were similar in IFS and OFS. Proportion of all 3 target cell types increase with age. History of balanitis/STIs significantly increased the number of target cells. |
| McCoombe, 2006 [6] |
30 subjects, 9 cadaversf (mean age 77.4 years) 21 subjectsf (mean age 28.9 years) 8 penile necropsy specimens† from Melbourne, Australia |
9 penile cadaveric specimens were obtained within 18 hours of death. 21 men were healthy and undergoing elective circumcision. |
HIV negative | LCs : 61.3 cells/mm2 in IFS 85.5 cells/mm2 in OFS 56.0 cells/mm2 in frenulum 41.0 cells/mm2 in glans OFS > IFS > frenulum > glans > urethral meatus LC dendritic processes in IFS came within 4.8µm of epithelial surface compared to 20 µm in OFS. CD4+ T cells: Found in epidermis, but predominant in dermisγ. Macrophages: Found in epidermis, but predominant in dermisγ. |
| Donoval, 2006 [23] | 39 subjects, 18-24 years from Kisumu, Kenya | Foreskins obtained from the randomised controlled trial in Kenya [3]. | 21 men without history of STI 19 men with a history of treated STI | Epidermis † Dermis † LC cells 1.23% 0.30 % CD4 T cells 0.08% 0.075% Macrophages 0.02% 0.04% LC: Mainly found in epidermisγ. CD4+ T cells: No difference in the median percentages between epidermis and dermisγ.Macrophages: Found predominantly in dermisγ. |
| Qi Qin, 2009 [11] |
80 subjects, 60 subjects , 2-7
years 20 subjects, 20-29 years from Hangzhou, China |
Group 1; circumcised for medical reasons related to UTI. Group 2; circumcised for cultural and cosmetic reasons. Adult men had no history of UTI. |
Not specified | LCs : Children UTI related 132.2 cells/mm2 in IFS, 131.7 cells/mm2 in OFS Non UTI related 87.5 cells/mm2 in IFS, 123.7 cells/mm2 in OFS Adults 53.7 cells/mm2 in IFS, 88.3 cells/mm2 in OFS OFS > IFS of adults and healthy boys. OFS = IFS in boys with a history of infection. CD4+ T cells: Not specified Macrophages: Not specified |
| Fischetti, 2009 [21] | Subjectsfy (number of subjects or age range not specified) from London, UK | Elective circumcisions after gender reassignment (after 6 weeks off hormonal therapy). | Not specified | LCs : 230 cells/mm2 in IFS 170 cells/mm2 in OFS Exclusively reside in epidermis. The distance to dendritic projections of LCs from the epidermis surface Glans > IFS = OFS. Average number of LCs greatest for glans> IFS > OFS. CD4+T cellsspan>: 260 cells/mm2 in IFS, 2.6 times higher than OFS.Typically present in dermis, infiltrate epidermis under inflammatory conditions. Average number of CD4+T cells greatest for glans> IFS > OFS. Macrophages: NoNot specified |
| Hirbod, 2010 [17] | 33 subjects, 18-24 years from Kisumu, Kenya | Foreskins obtained from the randomised controlled trial in Kenya [3]. | All men were negative for STI for a period of 3 months prior circumcision. | LCs: 150 cells/mm2 in both epidermis and dermis (60% in epidermis and 40% in dermis). Intraepithelial LCs localised as close as 24µm to the outer surface of IFS. CD4+ T cells: Abundant CD4+ cells present in dermisγ under the epidermis of foreskin function as early sites of viral replication. Macrophages: Exclusively found in dermisγ. |
| Ganor, 2010 [22] | Subjectsy (number of subjects not specified) 17-87years from Paris, France | Personal reasons or phimosis | Foreskins with history of infectious pathologies were not used. | LCs: IFS ≈ 580 cells/mmup>2, OFS ≈ 220 cells/mm2, and LC cells only found in epidermis of IFS. IFS >OFS CD4+ T cells: mainly in dermis, and about 2× concentration in IFS vs. OFS. IFS >OFS IFS OFS % CCR5 expressing LCs out of total LCs 20.9± 1.6 6.1± 3.1 % CCR5 expressing T cells out of total T cells 35.5± 11.4 5.3± 3.6 Macrophages: Exclusively found in dermis and the density in IFS=OFSγ. |
(IFS, inner foreskin; OFS, outer foreskin; LC, Langerhans cells; STI, sexually transmitted infection; UTI, urinary tract infection).
ϕage range not specified Ψ number of subjects not specified *percentages were based on the number of brown staining cells indicating a specific immunophenotypic marker divided by the total number of nucleated cells †The mean cell percentages were derived from the amount of cellular area staining positive per total cellular area in 5 fields covering the IFS containing 50%epithelium and 50%dermis within each field. γPrimary data not given.
Studies on tissue explants to examine early stages of HIV-1 transmission.
| STUDY | TISSUE SOURCE & METHODS | RESULTS & CONCLUSIONS |
|---|---|---|
| Patterson,2002 [7] | Tissue: foreskins circumcised for phimosis, balanitis, adhesions and redundant foreskins cervical tissue samples from removed uteri for benign conditions.Culture: Three 4.0-mm biopsies from the IFS and three from the OFS cultured for 1 day.Infection: Foreskins were infected with CCR5-using (R5) HIV-1Bal or the CXCR4-using (X4) HIV-1Lai for 1 day.Measuring of infectivity: Infectivity quantified using real-time quantitative polymerase chain reaction for HIV-1 pol DNA. | Susceptibility of human foreskin to HIV-1:Infection was predominant in CD4 T cells and LCs of IFS.Infection was below the level of detection in OFS. Adult foreskin infection was higher than in an infant (22 months) foreskin.Adult foreskin from those without past history of STI’s was 9 times more susceptible than cervical tissue. |
| Fischetti L, 2009 [21] | Tissue : Penile tissues( glans, urethra and foreskin tissues ) from gender reassignment patientsCulture: Tissues sized 2-3 mm2 cultured for 10 days. Infection: Cultured cells were exposed to HIV-1BaL or HIV-1Lai for 2 hours for infection. After resuspended in PHA medium overnight, the tissue explants were removed and cultured for 3 days.Measuring of infectivity: Assayed for HIV-1 P24 antigen level using ELISA from cultured supernatants harvested every 2-3 days. | Susceptibility of human foreskin to HIV-1:No significant differences in the level of HIV-1 infection between different tissue sites (glans, urethra, foreskin).No evidence to support enhanced susceptibility of IFS relative to OFS or glans. HIV-1 BaL productively infected all tissue sites, but no infection was detected with HIV-1 Lai. |
| Ganor Y, 2010 [22] | Tissue: Normal healthy foreskin tissues circumcised due to personal reasons or phimosis.Culture: 8mm diameter round foreskin tissues used as novel ex-vivo explant model with restricted access of virus to the non-epidermal side of either IFS or OFS.Infection: 1. With low and high cell free HIV-1 loads. 2. With PBMCs weakly or highly infected with HIV-1.Measuring infectivity: Quantified HIV-1 by p24 ELISA. | Susceptibility of human foreskin to HIV-1:HIV-1 enters IFS explants, but is trapped within (thick) keratin layer of OFS. HIV-1 infected cells form viral synapses with apical keratinocytes.HIV-1 transmission is more effective when foreskin is inoculated with HIV infected cells, not the cell free virus.LC-T cell conjugates permit transfer of HIV-1 from LCs to T cells.Exposure of IFS to HIV-1 infected cells results in efficient translocation of HIV-1.Seminal plasma mixed with cervical-vaginal secretions decreases HIV-1 translocation through IFS. |
IFS, inner foreskin; OFS, outer foreskin; LC, Langerhans cells STI, sexually transmitted infection.
Qin’s (2009) study, including preschool boys (some with a history of urinary tract infection) and adults (all without a history of UTI), reported that the keratin thickness was much greater in the IFS than in the OFS in adults, and in boys with a history of infection [11]. There was no significant difference in keratin thickness of inner and outer foreskin tissues in boys without a previous history of UTI. In this study, they demonstrated a close relationship between thinner keratin layer of the OFS and desquamation in adults and boys. Furthermore, they reported the keratin layer as thinner in boys’ foreskins compared with those of adults. Qin and colleagues attributed these differences to anatomical and functional characteristics of ethnic groups (foreskins of men of Asian origin are reported to have a thinner stratum corneum layer compared to foreskins of men of other origins [12, 13]) socioeconomic, hygienic and nutritional factors [11].
Additional to keratin layer thickness some have suggested that the skin’s barrier function also relies on components such as intercellular junctions. These junctions serve to regulate cell and epidermal growth and to protect the integrity of the epidermis [14]. Dinh and colleagues (2011), in their review, outline that expression of these proteins (intercellular junctions) can vary between epithelial tissues in different areas of the body, which explains the difference in level of protection in certain areas of the body compared to others [15]. Dinh and colleagues further demonstrated through their earlier studies the subtle differences in protein expression patterns of foreskin and cervical tissues, which may contribute to differences in movement of HIV between the female and male genital tract [15].
Distribution of Cellular Receptors for HIV in the Foreskin (Table 2)
Researchers have generally focussed on the foreskin epithelia as a likely site of entry for HIV. They have followed basic immunohistochemical methods using antibodies specific to unique antigens to visualise and enumerate the target cells for HIV in the penile epithelia and adjacent tissues. Accordingly, the cellular receptors for HIV in foreskin are most likely the CD4 (principal receptor for HIV-1) and CCR5 (co-receptor) bearing T cells and the CD1a+ Langerhans cells (LC). LCs are found among the epithelial cells in the squamous epithelium/epidermis [16]. Dendritic cells (DC), T cells and C-type Lectin Receptor (CLR) expressing CD68+ macrophages lie deeper in the dermis, but nonetheless are also likely cellular receptors for HIV [16, 17]. The role of the urethral mucosa as site of HIV entry has received less attention [18].
Although the methods of visualisation of target cells were similar, these studies have adopted different methodologies in enumerating target cells within foreskin tissues. While some of the studies counted cells in inner and outer foreskins, other studies compared 2 histological strata i.e. epithelium (epidermis including inner and outer) and submucosa (dermis). Furthermore, the counting method of cells also differed; some studies provided actual numbers while others provided cell count as a percentage or a proportion of all staining cells. This made the comparison of results difficult though we have standardised the results into similar units as much as possible.
One of the earliest studies published on cell distribution of foreskin tissues was carried out with the participation of 10 subjects and revealed no significant difference in LC concentration between inner and outer foreskins [19]. McCoombe and colleagues (2006), in contrast, found the highest density of LCs in the OFS followed, in descending order by the IFS, frenulum, glans penis and urethral meatus [6]. Qi Qin also reported that the majority of LCs located in the outer superficial layers of foreskin and that OFS contain significantly higher number of LCs than IFS in healthy boys and adults (there was no significant difference in LC density in the outer and inner foreskin of boys with a history of urinary tract infection). The study also found an increased density of LCs in the IFS of boys with a history of urinary infection compared with IFS of healthy boys [11]. Qi Qin et al. further demonstrated much higher LC density in boys’ foreskins than in adults’ foreskins [11]. Farbach and colleagues similarly demonstrated higher LC density in OFS than IFS through their studies [20].
In contrast to all of the above results, Patterson et al. (2002) observed that LCs and CD4+ T cells (previously activated memory CD4+T cells) are present at high densities in the IFS epithelium [7]. They identified CCR5 as the predominant co-receptor expressed on HIV-1 target cells and reported the dermis as the site for cells expressing highest level of CCR5 [7]. In 2009, Fischetti et al. from their penile explant tissue studies, demonstrated that LCs were highest in glans, followed by IFS and OFS at early stages of foreskin being removed from the body [21]. They observed changes in LC densities after culturing tissues for few days. Ganor and colleagues, who also used penile explant tissues for their studies, demonstrated higher number of LCs and CD4 cells in IFS than OFS [22]. In an another study of young Kenyan males at high risk of HIV exposure revealed that intra-epidermal LCs were localized as close as 24 µm to the outer surface of the IFS [17]. This was close enough for their dendrites to reach out and sample HIV particles from genital secretions of a sexual partner [17]. Abundant CD4+ T cells were also reported in the dermis of the IFS in this study [17].
Fahrbach et al. attempted to understand the dynamics of the immunologic environment in the male genital tract by examining target cell activity in the inner and outer foreskins in response to inflammatory cytokines [20]. They showed a significantly higher responsiveness of LCs and CD4+ T cells in the IFS compared to the OFS to some cytokines. They further demonstrated that LCs maintain dynamic responsiveness to external stimuli and that intact IFS has greater access to materials in its external environment than the OFS [20].
In Vitro Experiments of Human Explants Cultures for the Permeability for HIV (Table 3)
Three studies have been published on human genital explants tissue to study the initial steps of HIV-1 transmission [7, 21, 22]. Of those, two studies identified the IFS as the predominant portal of entry compared to OFS for HIV [7, 22] while the other study proved no enhanced susceptibility of one surface to the other [21].
Two studies out of 3 investigated the mechanism of HIV-1 entry into foreskin using high doses of cell free HIV-1 at time periods of more than 24 hours. The first study which used agarose sealed foreskin and cervical tissue explants showed evidence for different levels of permeability for different strains of HIV at different genital tissue sites [7]. In this study, after adjusting for the differences in target cell proportions, the infectivity for HIV- 1 of the adult IFS was several times greater than cervical tissue. Notably, there was no HIV-1 infiltration in the tissue taken from the OFS [7]. The authors further observed an extensive infection of both the CD4 T cells and LCs through CCR5 when HIV-1Bal isolate was introduced to the foreskin tissue explant cultures. An ineffective infection was noted with HIV-1Lai isolate in all tissue samples [10]. However, this study was questioned later for its sealing efficiency and polarisation of the system [8]. In 2009, Fischetti et al. designed their study using non-polarised foreskin tissue explants in which HIV-1 gained access to both apical and basal sides of the tissue surfaces. They conducted research on different tissue explants taken from glans, urethral meatus, urethra and foreskin (inner and outer) and showed no significant difference in terms of susceptibility to HIV infection [21]. The outer and inner foreskins were infected to a similar degree with CCR5 (but not CCR4) with cell-free HIV in their study [21].
In contrast, Ganor and colleagues (2010) used two novel models of human adult foreskin epithelium for their study [22]. One model consisted an ex vivo foreskins (inner or outer) explant placed on a permeable membrane in a two chamber system. In this model, polarised epidermal surface was created by gluing hollow plastic cloning ring cylinders tightly on to the epidermal surface. The other model consisted an in vitro reconstructed immuno-competent foreskins constructed by seeding primary inner and outer foreskin fibroblasts and keratinocytes together with immature LCs and DCs in the apical compartment of the 2 chamber system. By optimising culture conditions, they managed to create in vitro models that resemble natural structural and morphological characteristics of foreskins. They used both cells infected with virus and cell free virus in high and low concentrations for infection of explant tissues and measured the viral penetration through tissues in shorter time points. Using these models, Ganor et al. demonstrated an efficient HIV-1 transmission after 1 hour of polarized exposure of the IFS to mononuclear cells (such as macrophages and CD4 cells) highly infected with HIV-1 [22]. Highlighting the next steps in mechanism of infection, they showed how such contact leads to viral synapse formation with foreskin keratinocytes, resulting in HIV-1 budding and rapid capture, internalisation and transcytosis by epidermal LCs [22]. Accordingly, LCs play an active role in sampling HIV-1 at the foreskin and transferring to T-cells. Those infected T-cells then initiate the local expansion of a small population of HIV-1 infected cells within the dermis of foreskin, which is a prerequisite for HIV-1 dissemination and systemic infection [22] (Fig. 3). The authors did not observe HIV-1 transmission through OFS when they used cell-free virus for infection [22].
Foreskin Surface Area and HIV Transmission
Kigozi and colleagues (2009) studied the effect of foreskin surface area (measured after circumcision) in HIV transmission. They examined 965 foreskins for surface area, excised from men enrolled in the Rakai Community Cohort Study in Kenya [24]. They found the mean foreskin surface area (43.3 cm2) among men who seroconverted to HIV was significantly larger than that (36.8 cm2) among men who remained uninfected (P=0.01). The risk of HIV acquisition was significantly increased among men with foreskins in the upper quartile of surface area (45.6-99.8 cm2) compared with men in the lowest quartile (adjusted IRR, 2.37, 95% CI 1.05-5.31) [24].
“Wetness” Beneath the Foreskin
A study was conducted in 2006 among men at a STI clinic in Durban to examine the association between sub-preputial penile wetness and HIV transmission. This study used direct clinical examination and HIV prevalence as tools to measure the association [25]. Here, the degree of wetness of the glans observed after foreskin retraction was classified into 4 grades: dry, slight wetness, wet, or very wet, with or without smegma, by genital examinations performed by 3 physicians. Results of this study showed that in men assessed as having any level of penile wetness, HIV prevalence was 66.3% compared with 45.9% in those with no penile wetness, and after adjusting for other predictors for HIV and confounders the OR was 2.38 (95% CI, 1.42-3.97, P=0.001). Interestingly, the study included a number of circumcised men, and the prevalence of HIV infection among them (42.9%) was similar to that among uncircumcised men with a dry penis (45.9%) [25].
Microbiome of Inner Foreskin
Price and colleagues highlighted the idea that the anoxic microenvironment of the sub-preputial space may support pro-inflammatory anaerobes that can activate LCs to present HIV to CD4 cells in draining lymph nodes [26]. They identified 42 unique bacterial families, of which Pseudomonadaceae and Oxalobacteriaceae were the most abundant, irrespective of circumcision status. They further demonstrated in 12 HIV negative uncircumcised Ugandan men that circumcision was associated with a significant change in the overall microbiota (p=0.007). There was a significant decrease in putative anaerobic bacterial families 12 months after circumcision (p=0.014), mainly Clostridiales Family XI and Prevotellaceae which were uniquely abundant before circumcision. Within these families they identified a number of anaerobic genera previously associated with bacterial vaginosis such as Anaerococcus spp., Finegoldia spp., Peptoniphilus spp., and Prevotella spp. Thus, the reduction in putative anaerobic bacteria after circumcision may play a role in protection from HIV and other sexually transmitted infections (STI) [26].
HIV and Other STIs
The idea that mucosal disruption associated with ulcerative STI facilitates HIV transmission drew attention of researchers to investigate a possible interaction between HIV and other STIs. Cameron et al. (1989) showed that the acquisition of HIV was highly associated with having GUD, being uncircumcised, and having frequent contact with sex workers [27]. In their study, men who reported a single contact with sex workers, and who had seroconverted, all had genital ulcers. Galvin and Cohen (2004) in their extensive review demonstrated that persons with STIs that cause ulcers and inflammation are more vulnerable to HIV than healthy individuals [28]. According to Fleming et al. the adjusted risk ratio for HIV acquisition for a person with Genitourinary Ulcerative Disease (GUD) ranges from 2.2 to 11.3, whereas with non-ulcerative STIs it is 3-4 [29]. Dickerson et al. reported that such associations persisted in most cases even after adjusting for sexual behaviour and other confounding factors [30]. Weiss et al’s systematic review of MC and ulcerative STI strongly indicated that circumcised men were at lower risk of chancroid and syphilis. However, the review reported only a borderline statistically significance for HSV-2 [31]. In 2009, Metha et al. demonstrated in their study in Kisumu, Kenya, that MC did not reduce the risk of acquiring non-ulcerative STIs (N. gonorrhoeae, C. trachomatis, and T. vaginalis) [32]. The same authors later showed that circumcised men had fewer M. genitalium infections [33]. Anderson and colleagues in their review showed that Treponema pallidum, Haemophilus ducreyi, and Neisseria gonorrhoeae infections do enhance HIV transmission, and that all of those infection were less frequent after circumcision [18].
A 46% reduction in genital ulcer disease (GUD) [4] and a 28% reduction in HSV-2 acquisition from MC was observed in the MC trial in Rakai, Uganda [34]. Similarly a MC trial in Orange Farm, South Africa demonstrated a 30% reduction in HSV-2 incidence among participants [35]. Contrary to findings from the South African and Ugandan trials, Metha et al. demonstrated in 2012 that the protective effect of MC against HIV was independent of GUD and HSV-2, and MC had no effect on HSV-2 incidence [36].
According to Dinh et al. the reason for the disparity seen between the effect of male circumcision on viral and bacterial pathogens is not entirely clear, but likely relate to differences in routes taken during transmission (i.e., the squamous epithelia found in foreskin, glans, and shaft tissue versus the columnar epithelium of the urethra) [15].
Genital Mucosal Disruptions
Penile cuts, abrasions, and tears during sexual intercourse are presumed to be another potential mechanism that place uncircumcised men at increased risk of acquiring HIV through disruption of epithelial and mucosal barriers. O’Farell et al. suggested that such abrasions are more common, and elaborated that the mucosal discontinuity as a constant finding with poor standards of genital hygiene. They further suggested that rapid healing of those abrasions is delayed due to the moisture beneath the foreskin which provides an excellent niche for other STI pathogens [37]. Szabo and colleagues highlighted that the frenulum, which is a highly vascular area of penile skin, is more susceptible during sexual intercourse and a common site of the penis for the ulcerative lesions from STIs. Thus they suggested that MC reduces HIV-STI synergy by removing foreskins [38].
In contrast, in 2009, Mehta et al. reported that early sex after MC is not associated with increased HIV risk [39]. In a later publication based on a randomised controlled trial (RCT) in Kenya, Metha et al. demonstrated that self-reported penile coital injuries were common among healthy 18-24 years old young men, nevertheless, circumcised men were at lower risk for coital injuries (0.62 [95% CI: 0.56-0.70] compared to uncircumcised men [40]. The exact mechanisms by which such injuries may increase risk for STIs and HIV infection are yet to be investigated.
DISCUSSION
There is strong evidence that demonstrates male circumcision can reduce HIV acquisition in heterosexual men by around 60%, however the exact mechanisms of protection still remains unclear. This review summarises the existing research that is advancing knowledge about the mechanisms of protection from HIV afforded by male circumcision.
The first natural barrier found on foreskin is the outermost keratin layer (stratum corneum) which consists of dead keratin and a basal layer of live keratinocytes. Different studies have shown different measurements of the keratin layer of inner and outer foreskins making the evidence inconclusive. Some evidence supports the concept that the IFS is less keratinised than the OFS and consequentially provides easy access for the virus to enter into the submucosa, but other evidence shows that the OFS is equally or more thinly keratinised and offers no favourability for transmission of the virus. Moreover, there is evidence to that demonstrates inter-individual and intra-individual differences in foreskin keratin thickness as well as differences in keratin thickness among different races. One explanation for the contrasting results of keratin thickness is the different methodologies used in measuring the keratin thickness in different studies. For example, in the Qi Qin study the authors did not measure the thickness of the most superficial dead keratin layer when they measured keratin layer thickness [11]. Different tissue sampling (e.g. different distances to the tissue sample from the coronal sulcus), different processing methods, and possible underlying pathologies such as STIs of the tissue donors may also have contributed to the differences in results. The keratin layer which is easily swollen through exposure to water and its easily detachable nature from the stratum granulosum during fixation could be another factor responsible for the difference in measurements. Additionally, cadaver samples could have different keratin thickness. It is important to remember that variation in keratin thickness among races, between individuals and within individuals suggest that factors other than race work together to create keratin thickness differences; and more laboratory studies need to be performed to understand these factors using tissue samples from men in vulnerable populations.
There are different schools of thought regarding the protection provided by the keratin layer against HIV acquisition. One argument is that the superficial keratin layer is easily sloughed off; therefore an intact layer is unlikely to be found after sexual intercourse to provide any protection against HIV infection. Another argument is that HIV transmission through the oral mucosa, with a very thin keratin layer, is very inefficient [15].
There is a general agreement about the types of target cells present in the foreskin such as LCs, DCs, macrophages and T4 cells that bear cellular receptors of CD4, CCR4 and CCR5, although contrasting results have been published about the distribution of these cellular HIV targets in the inner and outer foreskin. Differences are not only regarding the cell location, but also the number of cells in different sites of penile tissues (Table 2). A plausible explanation for these differences would be the presence of possible underlying pathologies in tissue donors that can result in changes in the distribution of immunological cells in and around an inflammatory process. These different results could also be due to the difference in site of origin of the tissue from the intact foreskin used in the analyses. Fischetti et al. also suggest the possibility of surgical trauma induced cellular redistribution with alteration of target cell densities in penile tissues after removal as an explanation for the different results achieved in previous studies [21]. After reviewing the studies, we can conclude that there is general consensus on the types of HIV target cells present in foreskin tissues and that there are more cellular targets found in IFS than in the OFS.
Studies where infection of HIV into tissue explants was performed, simulating the actual biological process of infection in vitro, provide the best evidence to date for the tissue sites involved with the acquisition of HIV. Inner foreskin was demonstrated as the main area for viral entry in 2 out of 3 studies done on human explant tissues. While Patterson’s and Ganor’s studies demonstrated the IFS as the only susceptible tissue out of the two foreskin surfaces, Fishchetti and colleagues demonstrated that no significant difference between tissues in terms of susceptibility to HIV amongst all of the exposed areas of penis (glans, inner and outer foreskins). However, Fischetti et al. concluded that circumcision removes 2 out of 3 susceptible exposed areas of penis, therefore reducing the chance of virus coming in contact with susceptible target cells. Patterson and colleagues considered the OFS as equivalent to the penile shaft for comparison with the IFS of the penis, and concluded that penile shaft tissue also impermeable for infiltration by HIV-1 [7] on the basis that the penile shaft is covered by a keratinized stratified squamous epithelium similar to the OFS. Although this finding rejected a previous hypothesis that the penile shaft may act as an entry point for HIV in circumcised men, further research is needed to enhance evidence about HIV entry along the penile shaft [7]. Some have speculated that the route for HIV-1 infection in uncircumcised males could be through the epithelium of the glans, as it is protected by the foreskin and is thus likely to be less keratinized in adults than the glans of the circumcised penis. Szabo and Short examined the glans of seven circumcised and six uncircumcised men, and found the epithelia of both groups were equally keratinised [38]. They demonstrated that in circumcised males, only the distal penile urethra is lined with a mucosal epithelium and speculated that it is unlikely to be a common site of infection as it contains comparatively few LCs. Instead the authors suggested that the infection could occur through disruptions of the penile shaft epithelia caused by genital ulcer disease or trauma [38].
Patterson’s and Fischetti’s explant models had only a few hours of functional integrity and contained migratory immune cells activated by surgical procedures on explant tissues and had inefficient sealing of the edges of explant tissues to ensure the polarisation of HIV infection. Therefore those models supposed to have failed to maintain the stratified architecture or actual numbers of immune cells to simulate the natural situation in foreskins [8]. Ganor et al. managed to overcome those problems with their novel models and demonstrated more solid evidence on HIV transmission through the male genital tissues as described above. Ganor and collegues further demonstrated that interaction of HIV-infected mononuclear cells in partner’s sexual fluids with the IFS is the key to initiating infection, and further speculated in a later publication that this rapid process could be impeded by as yet ill-defined components activated while mixing of genital fluids [22]. Thus according to Ganor and colleague, removal of the foreskin, especially the inner aspect with circumcision eliminates a mucosal surface rich in HIV-1 target cells that serves as an efficient HIV-1 entry portal in men [22].
The observations in Kigozi et al. study strongly suggest that larger foreskin size is a risk factor for HIV acquisition in uncircumcised men [24]. These findings supported with Fischetti et al. explant tissue findings, in addition to the observational studies and RCTs, add plausibility again to the hypothesis that the foreskin is the main tissue of penis vulnerable to HIV acquisition. The increased risk of HIV acquisition among men with larger foreskin surface areas may be due to the presence of a larger number of HIV target cells in the IFS that is exposed to infected vaginal fluids during sexual intercourse. Men with larger foreskin surface areas may also be more vulnerable to trauma of the foreskin during sexual intercourse, increasing the risk of HIV acquisition. Kigozi and colleagues emphasized the implications of these findings for the surgical procedure of circumcision and suggested the need to minimize residual foreskin tissue after male circumcision [24].
O’Farrel et al’s study on subpreputial wetness provides better insights into another important factor that could contribute towards the HIV transmission through foreskin tissues [25]. According to this study, two third (66.3%) of the men who were infected with HIV had some amount of penile wetness as opposed to 45.9% who were infected with HIV but had no penile wetness (p=0.001). The authors have suggested explanations responsible for their findings; firstly, impaired healing of sexually acquired ulcers due to wetness in the prepuce, microulcerations caused by subprepusal balanitis due to wetness, enhanced adherence of infective HIV virions onto the HIV target cells of the IFS in the presence of wetness and finally, recruitment of more HIV target cells due to enhanced immune response by the wetness of the IFS [25]. This study did not assess the level of penile wetness of circumcised men adequately and the number of circumcised participants were few (n=55) compared to the number of uncircumcised participants (n=589).
Price et al. extended their study on the level of anaerobic bacteria in preputial space to learn the bacterial diversity in male genital mucosa [26]. They revealed a decrease in anaerobic bacteria after circumcision which may have related to the elimination of anoxic microenvironments under the foreskin [26]. Detection of these anaerobic genera in other human infectious and inflammatory pathologies suggests that they may mediate genital mucosal inflammation or co-infections in the uncircumcised state. Hence, according to authors, the decrease in anaerobic bacteria after circumcision may complement the loss of the IFS to reduce the number of activated LCs near the mucosal surface and the risk of HIV acquisition in circumcised men [26].
As well as the mechanisms discussed above, there is a body of evidence indicating that STIs that cause mucosal inflammation and ulcers contribute to the spread of HIV, by increasing infectiousness, susceptibility or both [28]. MC, which has been shown to be protective against such inflammatory and ulcerative STI acquisition (as in most of the studies mentioned above), could therefore, have a protective effect on HIV transmission.
Genital ulcers caused by STIs (on the glans and especially in the area of frenulum) and the associated inflammation are expected to increase the number of HIV susceptible/target cells locally. With the observation that the IFS is rich in CD4+ T-cells, macrophages and LCs, the presence of STIs affecting the IFS therefore further exacerbates the risk of HIV transmission through IFS by migrating immune cells [32]. With regard to non-ulcerative STIs, Mayer et al. held a similar view to Ganor et al. who suggested enhanced activity of CD4 cells after STIs (e.g. N. gonorrhoeae). Mayer et al. further emphasised the association between HSV-2 and HIV, whereby HSV-2 induces persistent expression of CCR5, which is a main co-receptor for HIV-1, making genital tissue vulnerable for HIV-1 even after treating for HSV-2 [36]. Apart from biological mechanisms, Mayer and Venketesh highlighted an epidemiological link between high HIV susceptibility of patients with STIs [36]. They proposed that STIs could be a marker of increased sexual risk behaviour and of contact with a HIV infected partner.
Another area of concern in the area of HIV prevention is the level of protection provided by MC for women. A RCT with participation of 922 uncircumcised men in Rakai, Uganda which was stopped early due to futility demonstrated that circumcision of HIV-infected men did not reduce HIV transmission to female partners over 24 months though the study did not assess longer-term effects [41, 42]. A meta-analysis of data from 6 longitudinal studies and 1 RCT also did not provide enough evidence for a direct effect of MC in reduction of HIV risk in women (summary relative risk 0·80, 95% CI 0·53-1·36) [43].
Beaten et al. demonstrated ~40% (statistically insignificant) reduction in the risk of acquisition of HIV by women from a circumcised male partner (hazard ratio 0.62, 5% CI 0.35-1.10, p=0.10). They reported no increased risk for the women among serodiscordant couples where male partner was seropositive and circumcised, however, they suggested a “potential” decreased risk from MC on male-to-female transmission of HIV-1 [44]. Hallete et al. in their publication based on mathematical modelling of data generated from two independent observational cohorts on the long-term effect of MC on male-to-female HIV transmission estimated that there would be an effective 46% reduction in the rate from 2 years after the MC operation [45]. A presentation at Conference on Retroviruses and Opportunistic Infections (CROI) 2014 revealed a statistically significant (p=0.004), 15% reduction in risk for women who had sex with only circumcised men compared to women who reported that some or all of their partners were uncircumcised [46]. This study was conducted in Orange farm, South Africa, the place that hosted the first RCT of MC for HIV prevention. Nevertheless, it was not clear in the study that this protection was due to direct effect of MC or the low HIV prevalence among circumcised men. Based on all the evidence, there could be a direct protection for women who have sex exclusively with circumcised men as well as indirect “herd immunity” for HIV infection for women from MC. However, this important relationship needs to be supported with further epidemiological and clinical research.
There are numerous studies reporting on HIV risk modification by MC for men who have sex with men (MSM). As early as 1993, it was shown that uncircumcised MSM had 2 fold increased risk of HIV infection (adjusted OR 2 (95% CI, 1.0-4.0) [47]. Later, a study by Buchbinder et al. (2005) demonstrated a similar 2 fold increase in risk of HIV acquisition for MSM associated with lack of circumcision [48] although the Population Attributable Ratio in their study population was relatively low (10.2).
Grulich et al. (2001) demonstrated no difference in MC status of men infected by receptive or insertive unprotected anal sex [49]. However, this study did not control for behavioural risk of participants. Based on cross-sectional data, Millet et al. (2007) found no evidence to support MC as a protective measure against HIV infection among black or Latino MSM [50]. Findings from a study in Seattle also suggested that MC did not have a significant impact on HIV or STIs acquisition among MSM [51]. A metanalysis of observational studies of MSM in 2008 further demonstrated lack of evidence for the protection by MC against HIV infection or other STIs [52]. The 2008 National HIV Behavioural Surveillance System cross-sectional survey conducted in 21 US cities among 5183 MSM not previously diagnosed with HIV infection, demonstrated that incarceration history, circumcision status, and sexual networks were not independently associated with HIV infection [53]. Gust et al. reanalysed a Phase III HIV vaccine clinical trial and reported that being uncircumcised does not confer a statistically significant increase in HIV infection risk among men who reported unprotected insertive anal sex with HIV positive partners [54].
According to Sanchez et al. circumcision did not have a significant protective effect against HIV acquisition among MSM from Peru and US, however, they suggested a possible reduced risk for men who were primarily insertive with their male partners [55]. Similarly, Templeton et al. (2009) demonstrated a significant reduction in HIV incidence among the Australian participants who preferred the insertive role in anal intercourse [56]. Later in a systematic review, Templeton et al. further demonstrated that circumcised MSM who predominantly take the insertive role in anal intercourse could be at a lower risk of HIV infection [57]. According to the Cochrane review in 2011, MC “may be” protective among MSM who practice primarily insertive anal sex, but the role of MC in the overall prevention of HIV and other STIs among MSM remains to be determined [58].
After reviewing the available literature, the authors have identified several research priorities in defining the mechanism/s how male circumcision reduces the risk of HIV acquisition. One priority is to analyse keratin thickness of inner and outer foreskins in live healthy men using physical properties inherent to the keratin layer such as optical absorbance. This could be done using non-invasive methods such as stratum corneum infrared densitometry [59]. Non- invasive measurements can improve measurements due to structural changes that occur in tissues once they are removed from the body or when the person is deceased. The authors believe that this would shed new light on measuring keratin thickness differences between inner and outer foreskins. Furthermore, assessment of the physical properties of resistance for viral entry using non-invasive methods such as trans-epithelial water loss, moisture content of the stratum corneum and skin surface pH provide perspectives beyond keratin thickness alone as a the principal mechanism of protection. On the other hand, quantitative research can be helpful to measure the exact relationship of HIV transmission with the amount of “wetness” of the preputial area which can be measured with newer methods such as moisture meters. Similarly, the effects of the microbiome of the penis in HIV transmission through male genital tissues need to be quantified. The limited research done to date needs strengthening to validate these observations. Finally, a more nuanced understanding of the mechanism of protection provided by MC against HIV transmission should be applied to an understanding of how modified forms of circumcision affect HIV transmission. Men in Papua New Guinea, for example, commonly practice traditional forms of penile cuttings which involve a longitudinal cut of the foreskin without foreskin removal [60, 61]. An understanding of whether these modified forms of MC that may transform the properties of the IFS have an effect on HIV transmission is needed by studying the changes on foreskin tissue components such as keratin thickness, target cell density and penile wetness, to further understand HIV transmission via male genital tissues and the mechanism of protection afforded by MC.
CONCLUSION
This review summarises research on the bio-physical mechanisms for protection provided by MC for men from heterosexual HIV transmission. Although there is substantial body of knowledge on the topic, there are still unresolved areas regarding the exact mechanism of protection. Undoubtedly, with all the evidence summarised above, this mechanism is complex with numerous factors working together to facilitate this well documented protection from HIV. Based on the evidence from the summarised studies, the mechanism of HIV transmission through the penile tissues stems as follows. When HIV-1 infected cells come into contact with foreskin, especially with IFS, they make synapses with the epithelial cells. This results in HIV-1 budding and subsequent capture by epidermal LCs through dendrites. This is followed by transfer of virus to T cells to initiate local expansion, HIV dissemination and systemic infection. The large surface area of the foreskin increases the chances of synapse formation by increasing number of contacts, while subprepusal wetness can facilitate the process by keeping the virus alive. This process is facilitated by abundant HIV target cells found in IFS and their higher responsiveness through altered cellular protein expression in response to external stimuli. Chemokines present in genital fluids further change the spatial distribution of HIV target cells (especially LCs) favouring connections with HIV infected cells. Furthermore, presence of concurrent STIs and microbiome under the prepuce and induced inflammation therein amass the target cells into dermal and epidermal tissues to facilitate the process. The closeness of target cells and their dendrites reaching closer to apical surface enhance the HIV infection process further. Physiological factors such as mechanical friction during sexual intercourse that cause micro-trauma can also provide easy access for the virus. Removal of foreskin by MC disrupts most of these mechanisms and helps achieve protection for men from sexual HIV transmission.
Investing in research to increase our understanding of the mechanisms of HIV transmission and protection against heterosexual HIV acquisition in men should be a priority that supplements the judicious implementation of MC programmes in high HIV prevalence settings.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
Declared none.


