All published articles of this journal are available on ScienceDirect.
Evaluating New Definitions of Acute and Early HIV Infection from HIV Surveillance Data
Abstract
Background :
The U.S. HIV staging system is being revised to more comprehensively track early and acute HIV infection (AHI). We evaluated our ability to identify known cases of AHI using King County (KC) HIV surveillance data.
Methodology :
AHI cases were men who have sex with men (MSM) with negative antibody and positive pooled nucleic acid amplification (NAAT) tests identified through KC testing sites. We used KC surveillance data to calculate inter-test intervals (ITI, time from last negative to first positive test) and the serologic algorithm for recent HIV seroconversion (STARHS). For surveillance data, AHI was defined as an ITI of ≤ 30 days and early infection as an ITI ≤ 180 days or STARHS recent result. Dates of last negative HIV tests were obtained from lab reports in the HIV surveillance system or data collected for HIV Incidence Surveillance.
Results :
Between 2005 and 2011, 47 MSM with AHI were identified by pooled NAAT. Of the 47 cases, 36% had ITI < 1 day, 60% had an ITI < 30 days, and 70% (95% CI=55-82%) had an ITI ≤ 6 months and would have been identified as early HIV infection. Of the 47, 38% had STARHS testing and 94% were STARHS recent.
Conclusion :
MSM with known AHI were not identified by proposed definitions of AHI and early infection. These known AHI cases were frequently missed by HIV surveillance because concurrent negative antibody tests were not reported. Successful implementation of the revisions to the HIV staging system will require more comprehensive reporting.
INTRODUCTION
Early identification of HIV infection is important from a public health perspective because individuals who are aware of their HIV status will change their behavior to reduce the risk of HIV transmission to others [1] and because initiation of antiretroviral therapy can decrease morbidity and mortality associated with HIV and also decrease transmission [2]. The recognition of acute HIV infection is particularly important, because people with acute HIV infection are highly infectious [3] and are the source of a significant proportion of ongoing transmission [4-6]. Furthermore, health department interviews (partner services) with recently infected individuals have a greater likelihood of case-finding compared to people with established infection [7]. For these reasons, several public health programs in the United States, including Seattle/King County, have developed pooled HIV nucleic acid amplification testing (NAAT) programs to identify antibody-negative persons with acute HIV infection [8, 9] and prioritize partner services for persons with acute HIV infection [10-12].
In 2008, the New York City Department of Health and Mental Hygiene expanded their case definition to include acute HIV infection, initially defined as any person not previously reported to surveillance with an HIV RNA level greater than 5000 copies/mL and a negative screening test within one month or observed seroconversion over a one month period [11, 13]. Over the last few years, the Centers for Disease Control and Prevention (CDC) also considered several revisions of the HIV surveillance case definition [14]. One of these revisions would expand the staging system to include Stage 0 HIV infection. The revision includes individuals with a positive HIV test within 180 days of a most recent HIV antibody test that was negative or indeterminate (http://www.cdc.gov/mmwr/preview/mmwrhtml/rr6303a1.htm).
Since 2003, the Public Health - Seattle & King County (PHSKC) HIV/STD NAAT Program has identified over 100 individuals with acute and early HIV infection. Since 2005, PHSKC has also participated in the HIV Incidence Surveillance Project (HIS) [15]. HIS collects remnant diagnostic sera to perform the serologic testing algorithm for recent HIV seroconversion (STARHS) test to classify newly diagnosed infections as recent (roughly within the past half year) or non-recent (infected approximately seven months or longer) [16]. We undertook this study to determine whether known cases of acute HIV infection could be identified through routine HIV surveillance mechanisms. We assessed the sensitivity of several proposed surveillance definitions of acute HIV infection (AHI) using inter-test intervals (ITI, the period between a last negative test and a first positive HIV test) and STARHS results when available.
METHODS
The PHSKC HIV NAAT Program
The PHSKC HIV NAAT program has been well-described [9, 17]. In short, the NAAT program screens EIA negative men who have sex with men (MSM) predominantly from public health sites through pooled HIV-1 RNA testing. For these analyses, NAAT positive cases were restricted to HIV cases identified by the program and who were residents of King County, Washington, and had tested confidentially between 2005 and 2011.
PHSKC Surveillance/Case Definitions
All seropositive HIV tests for confidentially tested King County residents (including EIA, WB, and HIV RNA) are reportable to the King County component of the National HIV Surveillance System (NHSS) case registry. Local and national laboratories passively report positive HIV tests; negative HIV screening results are not routinely reported. A last negative HIV test date is sought for all newly diagnosed HIV cases during initial case investigations. Last negative HIV test dates may be self-reported from a partner services interview or collected from a medical record review as part of HIV Incidence Surveillance (HIS). Dates of reactive home HIV tests and rapid HIV antibody tests are also obtained during these initial investigations.
For this analysis, we identified cases of acute HIV infection diagnosed through the pooled NAAT program with a single serum specimen that was HIV antibody-negative and NAAT positive. Inter-test intervals were calculated as previously described [15, 18]. We evaluated surveillance definitions that included 1) persons with a negative HIV antibody test obtained on the same day as the first positive HIV NAAT (AHI), 2) persons with a negative HIV antibody test obtained within 30 days of the first positive HIV NAAT or other positive HIV screening test (AHI), or 3) persons with a negative HIV antibody test obtained within 180 days of the first positive HIV NAAT or other screening test (acute or early HIV infection). STARHS results were obtained from HIS. The STARHS test requires a remnant specimen from a positive antibody test, thus specimens testing antibody negative and RNA positive (as in the NAAT program) are not eligible. The STARHS window period defining a recent infection (non-reactive) is approximately 153 to 162 days from infection [16, 19, 20]. Statistical analyses were performed using EpiInfo version 6.04 (CDC, Atlanta, GA, USA) and SAS version 9.2 (SAS Institute, Cary, NC, USA).
RESULTS
Between January 2005 and December 2011, 2152 HIV- infected King County residents were newly diagnosed and reported to PHSKC. Demographic and other characteristics of these persons are shown in Table 1. Individuals with STARHS and ITI were demographically similar to those without STARHS and ITI with respect to gender, age, race/ethnicity, and HIV risk. STARHS results were available for 1129 (53%) individuals, and 1284 (64%) had self-reported or documented dates for last negative HIV tests. During this time period, there were 47 MSM with acute HIV infection identified through the PHSKC NAAT program who were residents of King County and who tested confidentially.
Sociodemographic characteristics of newly diagnosed HIV cases by availability of STARHS test results* and inter-test intervals**. King County 2005 - 2011.
| Total N (%)*** | Have STARHS test* Yes N (%)*** | Have Inter-Test Interval ** Yes N (%)*** | |
|---|---|---|---|
| Sex | |||
| Male | 1893 (88) | 979 (87) | 1196 (93) |
| Female | 259 (12) | 150 (13) | 88 (7) |
| Age at HIV Diagnosis (Years) | |||
| 0 - 29 | 589 (27) | 364 (32) | 387 (30) |
| 30 - 39 | 701 (33) | 363 (32) | 419 (33) |
| 40 - 49 | 552 (26) | 258 (23) | 316 (25) |
| 50 - 59 | 237 (11) | 110 (10) | 129 (10) |
| 60+ | 73 (3) | 34 (3) | 33 (3) |
| Race/Ethnicity | |||
| Hispanic/Latino | 321 (15) | 184 (16) | 177 (14) |
| American Indian/AK native | 14 (1) | 6 (1) | 7 (1) |
| Asian & Pacific Islander | 119 (6) | 56 (5) | 54 (4) |
| Black | 406 (19) | 226 (20) | 165 (13) |
| White | 1235 (57) | 627 (56) | 845 (66) |
| Multiple | 56 (3) | 30 (3) | 36 (3) |
| Risk Category | |||
| Men who have sex with men (MSM) | 1384 (64) | 707 (63) | 980 (76) |
| Injection drug users (IDU) | 82 (4) | 52 (5) | 39 (3) |
| MSM/IDU | 181 (8) | 112 (10) | 133 (10) |
| Heterosexual | 217 (10) | 127 (11) | 73 (6) |
| Others | 288 (13) | 131 (12) | 59 (5) |
| Total (row%) | 2152 (100) | 1129 (53) | 1284 (60) |
* STARHS is the serological testing algorithm for recent HIV seroconversion and is used to distinguish individuals likely infected within the past half year versus those likely with longer standing infection
** nter-test interval is the period of time between a last negative HIV test and a first positive HIV test
*** Column percents
Of these 47 known cases, 17 (36%, 95% confidence interval [CI] 22-50%) men had a negative HIV test recorded as concurrent with their first positive HIV test (Fig. 1). An additional 11 men (60%, 95% CI 46-74%) had their last negative test recorded as within 30 days prior to their first positive test. Thus 60% (28/47) of the NAAT cases were correctly classified as AHI by routine HIV surveillance data. Thirty-three (70%, 95% CI 57-83%) of the 47 cases identified as antibody-negative/NAAT-positive had a prior negative test within 180 days of their HIV diagnosis (early HIV). STARHS results were available for 18 (38%) of the 47 known cases of acute HIV infection; results were non-reactive in 17 (94%). The 18th individual’s STARHS specimen was collected four months after his initial HIV diagnosis.
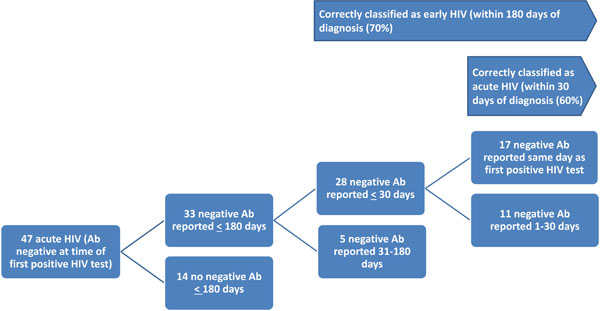
Inter-test intervals for 47 men who had sex with men diagnosed with acute HIV by pooled nucleic acid amplification testing: time from a last reported negative HIV antibody test (Ab) to HIV diagnosis based on reported HIV/AIDS Surveillance data, King County WA, 2005-2011.
Among all persons newly diagnosed with HIV during the analysis period, the median ITI was 12 months (mean=27 months, interquartile range 6 to 33 months). STARHS test results were available for 1129 (52%) of the study population, including 443 (39%) of whom were classified as recent and 686 (61%) were classified as non-recent infections (Table 2). There was rough agreement between ITI of a half year or less and the classification of STARHS recent. Sixty-eight percent (160/235) with STARHS results and with an ITI ≤ 6 months were classified as recent by STARHS (McNemar’s X2 p value <0.001).
Identification of recent and long term HIV infections by Serologic Testing Algorithm for Recent HIV Seroconversion (STARHS) and inter-test interval among newly diagnosed HIV cases in King County (2005 - 2011).
| Inter-Test Interval | Stage of Diagnosis by STARHS | Total* N | ||
|---|---|---|---|---|
| Recent N (%) | Long Term N (%) | Missing* N | ||
| Same date | 16 (94) | 1 (6) | 24 | 41 |
| 1-30 days | 16 (73) | 6 (27) | 25 | 47 |
| 1-6 months | 128 (65) | 68 (35) | 106 | 302 |
| > 6 months | 193 (36) | 339 (64) | 362 | 894 |
| Never tested before | 25 (20) | 98 (80) | 76 | 199 |
| Missing | 65 (27) | 174 (73) | 430 | 669 |
| Total | 443 (39) | 686 (61) | 1023 | 2152 |
* Includes 9 cases with insufficient quantity of remnant sera for a reliable STARHS test.
DISCUSSION
To effectively target and monitor HIV prevention and treatment efforts, it is important to be able to identify individuals with early and AHI [21-23]. Revisions to the CDC HIV surveillance case definition may help identify these individuals and also help allocate public health prevention resources to people with recent infection.
We found that the current surveillance definitions of AHI did not perform well in our population in King County, WA, missing 40% of a sample with known AHI (NAAT cases). The main reason for this poor performance was the lack of routine laboratory reporting for 64% of the negative HIV test results concurrent with the positive test result, i.e. negative antibody tests and positive p24 antigen or RNA tests that occurred on the same day. A change to mandatory laboratory reporting to include these negative tests will be critical for successful implementation of the revised definition and might be a relatively easy change to facilitate. Furthermore, a larger number of our known cases were classified as either acute or early because, likely for a variety of reasons, they had additional negative HIV tests proximal to their first positive test and our case investigators recorded these last negative tests on our surveillance forms. Additional persons could be appropriately classified with early or AHI if laboratory reporting also were to include recent negative HIV tests. Alternatively, more effort could be made to obtain this history as part of initial case investigations, which could then also capture recent negative point-of-care tests, at-home HIV tests, or other situations such as cross-jurisdictional testing which would likely not be included in laboratory-based surveillance.
Use of STARHS to identify and classify cases of early HIV infection has well-established limitations because the STARHS algorithm is known to produce both false recent and false non-recent results, although in aggregate these may balance out [24, 25]. Our data also show that use of STARHS may misclassify persons because antibody-negative specimens are not currently submitted for STARHS and cases of AHI will therefore not be included in this surveillance activity unless persons have blood drawn for subsequent HIV antibody testing. Additionally remnant specimens are not always available for STARHS testing. Our work is thus limited by a large amount of missing data. Some of these issues may be resolved with future incidence assays or incidence algorithms currently in development or with more widespread availability of data to estimate ITIs.
Another limitation of our analyses was that we conducted this initial test of the new surveillance definition using a subset of cases diagnosed with definitive AHI during this time period. Additional persons were diagnosed with AHI outside of the PHSKC pooled HIV NAAT program and were not included in our sensitivity analyses due to a lack of independence between the measurement and definition and because this would have required additional chart reviews to confirm their diagnoses. Similarly, we did not assess the specificity of the case definitions in this population, because that also would have required chart review.
In summary, HIV screening, incidence assays, and surveillance are currently in a state of flux. To better identify early HIV, the CDC is currently promoting HIV screening that combines antigen and antibody testing as an initial screening test, and a follow up test which allows for differentiation between HIV-1 and HIV-2 [26, 27]. In parallel, the CDC surveillance definition has the potential to better target partner services and behavior change interventions to highly infectious persons with recent HIV infection. However, in order for public health surveillance to make most effective use of this new classification, changes will be required to either laboratory reporting or disease investigation or preferably both.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
Declared none.


