RESEARCH ARTICLE
Comparative Evaluation of HIV-1 Neutralization in External Secretions and Sera of HIV-1-Infected Women
Qing Wei 1, Zina Moldoveanu 1, Wen-Qiang Huang 1, Rashada C Alexander 1, 2, Paul A Goepfert 3, Jiri Mestecky*, 1, 4, 5
Article Information
Identifiers and Pagination:
Year: 2012Volume: 6
First Page: 293
Last Page: 302
Publisher ID: TOAIDJ-6-293
DOI: 10.2174/1874613601206010293
Article History:
Received Date: 27/8/2012Revision Received Date: 31/10/2012
Acceptance Date: 6/11/2012
Electronic publication date: 28/12/2012
Collection year: 2012

open-access license: This is an open access article licensed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
Abstract
Objectives:
Although human immunodeficiency virus type 1 (HIV-1)-specific antibodies are detectable in external secretions by ELISA and western blot (WB), the presence of HIV-1 neutralizing antibodies is difficult to evaluate due to the low levels of immunoglobulins (Ig) and the presence of humoral factors of innate immunity. The objective of this study was to determine virus neutralization activity and the relative contribution of HIV-1-specific antibodies of various isotypes to virus neutralization in serum/plasma samples, cervicovaginal lavages (CVL), and rectal lavages (RL).
Design:
Serum/plasma, CVL, and RL samples were examined by ELISA, WB and HIV-1 neutralization assays. Selected samples were Ig depleted and analyzed for virus neutralization.
Results:
IgG specific for three HIV-1 ENV antigens was detected in all serum/plasma samples, while IgA to at least one ENV glycoprotein was found at the low levels in 95% samples. Serum/plasma samples had the ability to neutralize at least one of three clade B and two clade C viruses. The neutralizing titers were reduced significantly or became undetectable after IgG removal. In corresponding CVL and RL, HIV-1 ENV-specific IgG antibodies were readily detected compared to IgA. Furthermore, IgG in CVL had greater ability than IgA to reduce virus infectivity. The difference in HIV-1 neutralization before and after Ig depletion was not observed in RL, implying that innate humoral factors were involved in anti-HIV-1 activity.
Conclusions:
Results demonstrate that HIV-1-specific neutralizing antibodies are almost exclusively of the IgG isotype in serum/plasma and CVL samples. HIV-1-specific binding antibodies detected in RL are not responsible for neutralization activity, suggesting that the antibody-mediated virus neutralization in external secretions should be verified by means of a selective depletion of Ig.
INTRODUCTION
Recent studies highlight the importance of human immunodeficiency virus type 1 (HIV-1) neutralizing antibodies as correlates of protection against HIV-1 infection [1-4]. The direct protective role of neutralizing antibodies was most convincingly demonstrated in female macaques, which resisted vaginal challenge with simian HIV (SHIV) after systemic injection or intravaginal applications of broadly HIV-1 neutralizing antibodies of the IgG isotype [5-12]. It appears that in primates, IgG from the circulation is transported by a receptor-mediated mechanism into the female genital tract secretions [13] and contributes to approximately one-half of the IgG pool in the secretions. The remaining IgG is produced locally by the plasma cells present in genital tract mucosa [14-16]. In sharp contrast to all other external secretions, such as those of the intestinal tract, genital tract secretions contain IgG, and not IgA, as the dominant Ig isotype [17-21]. This point is of considerable importance because with the exception of the genitourinary tract, IgA is by far the dominant isotype in all other external secretions, including intestinal secretions. IgG in the intestinal tract is found only in small amounts and may be derived from the circulation or produced locally by plasma cells in the subepithelial lamina propria [16-21]. Moreover, humoral immune responses in serum/plasma, and in all external secretions after infection or immunization with HIV, are dominated by IgG; IgA responses are either absent or present at low levels [17-22]. Consequently, the two main mucosal sites of HIV-1 infections, the genital and intestinal tracts, display remarkable immunological differences with respect to the levels and dominance of Ig isotypes, selective Ig transport mechanisms, and immunization routes that are effective in the induction of antibodies [21]. Anti-HIV activity has been detected in mucosal secretions, including cervicovaginal and rectal lavages (CVL and RL), and the inhibition of HIV-1 infectivity can be caused by HIV-1-specific antibodies or by innate humoral factors [23-30]. Although neutralizing HIV-1 specific IgA antibodies have been reported in CVL samples from HIV-1-exposed seronegative women (HESN) [31, 32], the discrepancy in results was reported when highly similar cohorts were studied by other investigators [20, 22, 33, 34]. Nevertheless, HIV-1 neutralization activity in CVL of HESN and HIV-1-infected women was either not detectable or present at the low levels [22]. It is conceivable that the weak neutralization activity is not mediated by antibodies. Therefore, removal of Ig by immunosorbtion is required to prove unequivocally that the neutralization is indeed antibody-dependent. The purpose of this study was to ascertain the capacity of antibodies of the IgG or IgA isotype to neutralize HIV-1 in serum/plasma, CVL, and RL samples of HIV-1-infected women. To do so, we determined the levels of total Ig, the presence of HIV-1-specific binding antibodies of the IgG and IgA isotypes, the ability to neutralize HIV-1, and the relative contribution of the IgG and/or IgA isotypes to virus neutralization.
MATERIALS AND METHODS
Subjects and Specimens
All 63 HIV-1 infected women (Table 1) were recruited from the 1917 Clinic at the University of Alabama at Birmingham (UAB). At entry of the study, the average of absolute CD4+ T-cell count and plasma viral load was 601/µl and 30,860 vRNA copies/ml, respectively. Thirteen of the subjects had a level below the limit of detection (<50 vRNA copies/ml). The majority of patients (93%) had been diagnosed as having HIV-1 for at least 1 year. Four of the subjects were identified as HIV-1 positive for 2-7 months. After obtaining written and informed consent approved by the local institutional review board and biosafety committee, serum/plasma, CVL, and RL samples were collected. Freshly obtained serum and/or acid citrate dextrose-processed plasma samples were aliquoted and frozen at -80°C. Serum/plasma samples were heat-inactivated at 56°C for 1 h before being used in assays. The difference in HIV-1 neutralization activity between serum and plasma was not observed by us and another group [35]. The CVL samples were obtained by flushing the cervix and vagina with 5 ml of sterile saline; the washes were collected from the posterior vaginal vault into tubes containing protease inhibitors (Sigma-Aldrich, St. Louis, MO) [36]. The RL samples were collected after the instillation of 50 ml of sterile saline into the rectum; the fluids were retained for up to 5 min, and then expelled into a collection device with protease inhibitors [36]. Both CVL and RL samples were then centrifuged, aliquoted, and stored at -80°C until assayed.
Demographic of HIV-1-Infected Women
| Parameter | Average | Range |
|---|---|---|
| Age | 41 | 18-67 |
| Absolute CD4/µl | 601 | 16-1,551 |
| Plasma vRNA (copies/ml) | 30,860 | <50-792,580 |
| Years infecteda | 7.19 | 0.17-22 |
| Race | African-American 79% | Caucasian 21% |
a Number of years since the time of HIV-1 infection diagnosis.
Total Ig Levels in Various Fluids
| Fluid | na | IgA (ug/ml) (Mean ± SD) | IgG (ug/ml) (Mean ± SD) | IgM (ug/ml) (Mean ± SD) |
|---|---|---|---|---|
| Serum/plasma | 27b | 2,405 ± 1,289 | 18,632 ± 9,183 | 1,479 ± 875 |
| CVL | 14 | 6 ± 7 | 47 ± 62 | 1 ± 3 |
| RL | 14 | 72 ± 62 | 8 ± 13 | 17 ± 19 |
a n=Number of samples assayed for total Ig levels.
b Ig levels were determined in samples exhibiting relatively higher virus neutralizing titers.
HIV-1 ENV-Specific Binding Antibodies Determined by WB
| Fluid | na | IgG | IgA | ||||
|---|---|---|---|---|---|---|---|
| gp160 | gp120 | gp41 | gp160 | gp120 | gp41 | ||
| serum/plasma | 63 | 63 (100%)b | 63 (100%) | 63 (100%) | 59 (94%) | 39 (62%) | 54 (86%) |
| CVL | 11 | 11 (100%) | 11 (100%) | 11 (100%) | 11 (100%) | 9 (82%) | 8 (73%) |
| RL | 11 | 9 (82%) | 8 (73%) | 9 (82%) | 8 (73%) | 0 (0%) | 1 (9%) |
a n=Number of samples tested.
b Results are expressed as the number of samples that tested positive with the percentage in parentheses.
HIV-1 Antigen-Specific IgG or IgA Antibodies Determined by WB
| Patient Code | Fluid | IgG | IgA | ||||
|---|---|---|---|---|---|---|---|
| ENV (gp160, gp120, gp41) | POL (p66, p51, p31) | GAG (p55, p24, p17) | ENV (gp160, gp120, gp41) | POL (p66, p51, p31) | GAG (p55, p24, p17) | ||
| WARO | S/Pa | 3b | 3 | 3 | 3 | 3 | 3 |
| CVL | 3 | 3 | 3 | 3 | 2 | 3 | |
| RL | 3 | 3 | 3 | 1 | 1 | 1 | |
| JOTA | S/P | 3 | 3 | 3 | 3 | 3 | 2 |
| CVL | 3 | 3 | 3 | 3 | 1 | 1 | |
| RL | 3 | 3 | 3 | 1 | 2 | 1 | |
| THTO | S/P | 3 | 3 | 3 | 3 | 3 | 1 |
| CVL | 3 | 3 | 3 | 2 | 2 | 1 | |
| RL | 3 | 3 | 3 | 2 | 2 | 1 | |
| JODE | S/P | 3 | 3 | 3 | 3 | 3 | 3 |
| CVL | 3 | 3 | 3 | 3 | 3 | 1 | |
| RL | 3 | 3 | 3 | 1 | 2 | 2 | |
| GRLI | S/P | 3 | 3 | 3 | 3 | 3 | 3 |
| CVL | 3 | 3 | 3 | 3 | 1 | 3 | |
| RL | 3 | 2 | 3 | 0 | 0 | 0 | |
| SIDA | S/P | 3 | 3 | 3 | 2 | 1 | 2 |
| CVL | 3 | 3 | 3 | 1 | 1 | 0 | |
| RL | 2 | 2 | 1 | 0 | 1 | 0 | |
| HUTA | S/P | 3 | 3 | 3 | 3 | 2 | 3 |
| CVL | 3 | 3 | 2 | 2 | 1 | 1 | |
| RL | 3 | 3 | 2 | 1 | 2 | 2 | |
| HAAL | S/P | 3 | 3 | 3 | 3 | 3 | 3 |
| CVL | 3 | 3 | 2 | 3 | 2 | 2 | |
| RL | 3 | 2 | 2 | 1 | 1 | 1 | |
| JOME | S/P | 3 | 3 | 3 | 3 | 2 | 1 |
| CVL | 3 | 3 | 2 | 3 | 2 | 0 | |
| RL | 0 | 0 | 0 | 1 | 0 | 0 | |
| MIUR | S/P | 3 | 3 | 3 | 3 | 2 | 1 |
| CVL | 3 | 3 | 1 | 3 | 0 | 0 | |
| RL | 3 | 0 | 0 | 0 | 1 | 0 | |
| PEDO | S/P | 3 | 3 | 3 | 1 | 2 | 0 |
| CVL | 3 | 3 | 1 | 2 | 2 | 0 | |
| RL | 0 | 0 | 0 | 1 | 0 | 0 | |
a S/P=serum/plasma.
b Number of bands representing HIV-1 ENV, POL, GAG proteins detected in serum/plasma, CVL, and RL samples of 11 HIV-1-infected women by WB.
HIV-1 Neutralization Activity in Serum/Plasma Samples
| na | Clade B | n | Clade B | n | Clade B | n | Clade C | n | Clade C |
|---|---|---|---|---|---|---|---|---|---|
| HIV-1SF162 | HIV-1NL4.3 | HIV-1YU2 | HIV-1TV1 | HIV-192BR025.09 | |||||
| 1 | <30b | 24 | <30 | 38 | <30 | 1 | <20 | 1 | <20 |
| 12 | 30-900c | 20 | 30-270 | 9 | 30-270 | 2 | 21-270 | 2 | 21-270 |
| 41 | 901-8,100 | 14 | 271-2,430 | 9 | 271-810 | 2 | 271-400 | 1 | 271-400 |
| 8 | 8,101-72,900 | 2 | 2,431-7,290 | 3 | 811-2,430 | 1 | 401-640 | 1 | 401-640 |
| 1 | 120,330 | 3 | 7,291-10,000 | 4 | 2,431-5,660 |
a n=Number of samples.
b IC50 lower than starting dilution.
c Results are expressed as the range of IC50.
Efficiency of Total IgA and/or IgG Depletion
| Fluid | IgA Depletion (%)a (Mean ± SD) | IgG Depletion (%) (Mean ± SD) |
|---|---|---|
| Serum/plasma | 98.55 ± 1.75 | 99.83 ± 0.23 |
| CVL | 88.86 ± 15.88 | 99.37 ± 0.46 |
| RL | 91.15 ± 9.7 | 95.06 ± 3.64 |
a Percent depleted IgA and/or IgG was calculated as described in Materials and Methods section.
HIV-1 Neutralization in Intact and Ig-Depleted Serum/Plasma Samples
| Patient Code | HIV-1SF162 | HIV-1NL4.3 | HIV-1YU2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S/Pa | IgA-Db | IgG-D | (IgA+IgG)-D | S/P | IgA-D | IgG-D | (IgA+IgG)-D | S/P | IgA-D | IgG-D | (IgA+IgG)-D | |
| HAAL | 25,331 | 4,645 | 846 | 20 | 2,416 | 1,695 | <20 | <20 | 1,703 | 2,068 | <20 | <20 |
| CRNA | 4,220 | 2,256 | 537 | <40 | 722 | 400 | 20 | <20 | 134 | 84 | <20 | <20 |
| DASH1c | 5,773 | 2,587 | ND | <20 | 80 | 209 | ND | <20 | <20 | <20 | ND | <20 |
| DASH2c | 6,457 | ND | 98 | <20 | 157 | ND | <20 | <20 | 135 | ND | 68 | <20 |
| DUTA | 10,748 | 5,671 | 69 | <40 | 359 | 227 | <20 | <20 | 262 | 46 | <20 | <20 |
| FOBId | 3,194 | 1,933 | 1,638 | 1,056 | 11,972 | 2,388 | 5,534 | 1,846 | 2,937 | 2,004 | 1,622 | 1,268 |
| HALE | 2,922 | 1,205 | 846 | <20 | 357 | 117 | <20 | <20 | 194 | <20 | <20 | <20 |
| HUCA | 35,469 | 20,996 | 8,628 | 4,037 | 527 | 805 | 68 | <20 | 79 | 21 | <20 | <20 |
| JOOL | 15,350 | 10,178 | 40 | <40 | 332 | 537 | 126 | <20 | 443 | 381 | <20 | <20 |
| KEMA | 10,168 | 3,189 | <40 | <40 | <20 | 37 | <20 | <20 | 944 | 945 | <20 | <20 |
| WARO | 146,312 | 44,937 | 271 | <40 | 713 | 676 | <40 | <40 | 149 | <40 | <40 | <40 |
| THTO | 2,829 | 2,384 | <40 | <40 | 119 | 121 | <20 | <20 | <20 | <20 | <20 | <20 |
| MIURd | 18,828 | 13,902 | 2,966 | 927 | 6,151 | 4,260 | 4,183 | 1,247 | 3,937 | 2,525 | 2,831 | 852 |
| JOTA | 5,512 | 4,288 | <40 | <40 | 819 | 551 | <20 | <20 | 377 | 176 | <20 | <20 |
| JOMEd | 2,520 | 686 | 310 | 86 | 1,196 | 951 | 329 | 217 | 761 | 480 | 460 | 190 |
| PEDO | 2,506 | 2,819 | 40 | <40 | 205 | 331 | <20 | <20 | 333 | 238 | 224 | 25 |
| JODEd | 120,330 | 37,419 | 3,314 | 730 | 12,884 | 4,893 | 4,275 | 1,298 | 4,347 | 1,686 | 2,065 | 514 |
| HUTA | 2,830 | 935 | 868 | <40 | 799 | 911 | 277 | 237 | 354 | 190 | 125 | 44 |
| GRLI | 984 | 427 | <40 | <40 | <20 | <20 | <20 | <20 | <20 | <20 | <20 | <20 |
| SIDA | 5,266 | 3,356 | 615 | <40 | 439 | 201 | 69 | 37 | 72 | 74 | 80 | 24 |
a S/P=serum/plasma.
b IgX-D represented IgA, IgG, or both depleted from serum/plasma samples.
c Plasma samples from DASH were collected twice, 7 months apart.
d ART patients.
HIV-1-Specific IgA ENV Binding Antibodies in Serum/Plasma Samples
| Patient | HAAL | CRNA | DASH | DUTA | FOBI | HALE | HUCA | JOOL | KEMA | WARO | THTO | MIUR | JOTA | JOME | PEDO | JODE | HUTA | GRLI | SIDA |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Code ENV | |||||||||||||||||||
| gp160 | 2+a | + | 3+ | + | + | ± | ± | + | + | + | + | 2+ | + | + | ± | + | + | 2+ | - |
| gp120 | + | + | + | + | ± | ± | -b | - | + | + | ± | + | ± | + | - | + | - | ± | - |
| gp41 | 3+ | ± | 3+ | + | ± | - | - | + | + | 2+ | 2+ | 2+ | 2+ | + | - | + | ± | 3+ | ± |
a Plus(+) indicates samples were positive for HIV-1 ENV proteins. The relative intensity of bands was recorded as +, 2+, and 3+ (greatest).
b Dash(-) indicates that the sample tested negative.
HIV-1 Neutralization by CVL and RL Before and After Ig Depletion
| Patient Code | Fluid | HIV-1SF162 | HIV-1NL4.3 | HIV-1YU2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||||
| CVL/RL | IgA-D | IgG-D | (IgA+IgG)-D | CVL/RL | IgA-D | IgG-D | (IgA+IgG)-D | CVL/RL | IgA-D | IgG-D | (IgA+IgG)-D | ||
|
|
|||||||||||||
| WARO | CVL | 336 | 342 | 25 | 13 | 53 | 45 | 16 | 14 | 38 | 24 | 15 | 10 |
| RL | 29 | 29 | 15 | 12 | 20 | 20 | 18 | 14 | 17 | 16 | 14 | 10 | |
|
|
|||||||||||||
| HUTA | CVL | 17 | 12 | 10 | <10 | 32 | 19 | 21 | <10 | <10 | <10 | <10 | <10 |
| RL | 409 | 1296 | 402 | 684 | 131 | 149 | 111 | 100 | 149 | 222 | 171 | 117 | |
|
|
|||||||||||||
| PEDO | CVL | 46 | 25 | 22 | <10 | 47 | 33 | 36 | 27 | 56 | 31 | 60 | 20 |
|
|
|||||||||||||
| GRLI | RL | 17 | 15 | 16 | 13 | 50 | 42 | 43 | 51 | 16 | 13 | 13 | 10 |
ELISA for Total Ig
The concentration of total Ig in serum/plasma, CVL, and RL samples was determined by ELISA as described previously [17, 18, 22]. Briefly, duplicates of 2-fold serially diluted samples and a human Ig reference serum (pool of calibrated human sera, LiquichekTM Immunology Control, BioRad, Hercules, CA) were added to 96-well microplates (Nalge Nunc International, Rochester, NY) coated with goat F(ab’)2 anti-human IgA, IgG, or IgM (Jackson ImmunoResearch Laboratories, West Grove, PA). The captured Ig was detected after consecutive incubations with biotin-labeled goat F(ab’)2 specific for human IgA, IgG, or IgM (Geneway Biotech. Inc., San Diego, CA), horseradish (HRP)-labeled ExtrAvidin (Sigma-Aldrich), and the peroxidase substrate (O-phenylenediamine-H2O2, Sigma-Aldrich). The color reaction was stopped with 1 M sulfuric acid. The absorbance was measured in an EL312 Bio-Kinetics microplate reader (Bio-Tek Instruments Inc., Winooski, VT) at 490 nm. Total Ig levels were calculated by interpolating the optical densities on calibration curves constructed using the Delta Soft 3 computer program (BioMettalics Inc., Princeton, NJ), as previously described [37].
HIV-1-Specific Antibodies
The presence of HIV-1-specific antibodies of the IgA or IgG isotype was determined by WB adapted from a previously described method [17, 18, 22, 38, 39] using commercial HIV-1 strips (Cambridge Biotech, Worchester, MA). The strips contain 9 HIV-1 gene products derived from HIV-1IIIB-infected H9 T cells. These proteins are identified as gp160, gp120, p66, p55, gp41, p51, p31, p24, and p17. Strips were incubated overnight at 4°C with 1:500 diluted serum/plasma samples or 1:20-1:50 diluted CVL and RL samples. High background staining was observed when the dilution of CVL and RL collected from either infected or uninfected individuals was lower (e.g., 1:2-1:10). This was attributed to nonspecific absorption of mucosal proteins and glycoproteins [18]. Strips were washed, then incubated with biotinylated F(ab’)2 fragments of goat anti-human IgA or IgG (Geneway Biotech. Inc., San Diego, CA), followed by ExtrAvidin alkaline phosphatase conjugate (Sigma-Aldrich), and finally developed with alkaline phosphatase substrate: p-nitro blue tetrazolium chloride enhanced 5-bromo-4-chloro-3-indolyl phosphate (Bio-Rad). Scoring of band intensity (from 0 to 3+) was done as previously described [17, 18, 22, 39].
Cell Cultures
The TZM-bl cell line (NIH ARRRP catalog no. 8129) and the human embryonic kidney cell line 293T (ATCC CRL-11268) were both maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS), L-glutamine (2 mM), penicillin (100 U/ml), and streptomycin (100 µg/ml). TZM-bl cell is a genetically engineered HeLa cell clone that expresses CD4, CXCR4, and CCR5, and contains Tat-responsive reporter genes for firefly luciferase (Luc) and Escherichia coli β-galactosidase under regulatory control of an HIV-1 long terminal repeat (LTR) [40, 41]. Cultures were incubated at 37°C in a humidified 5% CO2-95% air environment. Cell monolayers were split at 1:10 ratio by treatment with 0.25% trypsin in 1 mM EDTA solution (Invitrogen) when cells reached at about 90 percent confluency.
Preparation of Virus Stocks
HIV-1NL4.3 and pseudovirus (HIV-1SF162, HIV-1YU2, HIV-1TV1, and HIV-192BR025.09) stocks were generated by transfecting HIV-1NL4.3 full-length plasmid DNA (16 µg) or rev/env expression plasmid (5 µg) and an env-deficient HIV-1 backbone vector (pSG3Δenv 10 µg) into exponentially dividing 293T cells using FuGENE 6 according to the manufacturer’s protocol (Roche Applied Science, Indianapolis, IN). Virus-containing culture supernatants were harvested 60 h post-infection, centrifuged at 3,000 rpm for 10 min, passed through 0.22 µm filter, and frozen at -80°C. The newly generated virus stocks were subsequently used in the infectivity assay.
Virus Infectivity Assay
TZM-bl cells were seeded at a concentration of 104 cells/well in 96-well flat-bottom culture plates (BD Biosciences, San Jose, CA) 1 day prior to infection. Virus stock was serially diluted (three-fold dilutions) in 240 µl of complete medium with 20 µg/ml DEAE Dextran in separate Nunc 96-well MicroWell plates (Nalge Nunc International, Rochester, NY). 100 µl of serially diluted virus was added to each well (duplicated at each dilution) after the medium in the culture plates was discarded. After a 48 h incubation, the medium was removed and 100 µl of 1 x lysing buffer (Promega, Madison, WI) was added to each well. Plates underwent two freeze-thaw cycles to allow complete cell lysis. 20 µl of cell lysate was then transferred to 96-well white solid plates (Thermo Fisher Scientific) to measure luminescence in a Victor 2 luminometer (Perkin-Elmer Life Sciences, Shelton, CT) after 100 µl of Luciferase Assay System substrate (Promega) was automatically injected into designated wells. Dilutions of virus stocks that yielded relative light units (RLU) from 150,000 to 250,000 on TZM-bl cells were identified and employed in the neutralization assay.
Neutralization Assay
Neutralization was measured as the reduction in Luc reporter gene expression after a single round of virus infection in TZM-bl cells [42, 43]. Briefly, freshly trypsinized cells were cultured overnight in 96-well flat-bottom culture plates at a density of 104 cells per well. Serial dilutions of test samples were prepared in 120 µl of complete medium in separate Nunc 96-well plates. Virus stocks were diluted (1:1200 for HIV-1NL4.3, 1:240 for pseudoviruses) in 120 µl of complete medium with 40 µg/ml of DEAE Dextran and added to each well of the test sample plate, yielding a total volume in each well of 240 µl. Two sets of control wells were also included (each with at least 6 wells). One set contained cells plus virus (virus infectivity control), and another set contained cells only (background control). The virus-sample mixtures were incubated at 37°C for 1 h. The medium from the cell plates was discarded, and then 100 µl of the virus-sample mixtures was transferred into the corresponding wells (duplicate for each dilution). After a 48 h incubation, the luciferase activity expressed as RLU was measured as previously described for the virus infectivity assay. The reduction of viral infectivity was calculated by comparing the average RLU in wells cultured with virus alone after the subtraction of the background RLU. The highest dilution of a sample that inhibited viral infection by 50% was considered the neutralization titer (IC50). Indinavir (NIH ARRRP catalog no. 8145), a protease inhibitor, was added to a set of HIV-1NL4.3 control wells at a final concentration of 1-2 μM, which is sufficient to inhibit nearly all secondary infections [44]. The difference in the mean RLU from the HIV-1NL4.3 control and from HIV-1NL4.3 with Indinavir were not statistically significant (p=0.10), indicating that HIV-1NL4.3 underwent only a single round of replication in TZM-bl cells during the 48 h incubation.
Depletion of IgG and IgA
IgA and/or IgG was removed by incubating serum/plasma, CVL, and RL samples with immobilized Jacalin (Pierce, Rockford, IL) or Protein G Sepharose (GE Healthcare Bio-sciences Corp., Piscataway, NJ), respectively. The amount of Jacalin or Protein G to be used was calculated according to manufacturer’s indicated binding capacity for IgA or IgG. However, an approximately 20% excess of Jacalin or Protein G was used for Ig depletion in order to ascertain the complete removal of the selected Ig. Levels of total Ig in samples before and after depletion were determined by ELISA. The percentage of Ig depletion was calculated as follows: 100-[(Ig concentration after depletion / Ig concentration before depletion) x 100].
RESULTS
Levels of Total Ig in Serum/Plasma, CVL, and RL Samples
IgA was the dominant total Ig isotype in RL, while IgG was dominant in CVL as well as in serum/plasma samples (Table 2). Although the pattern of IgG>IgA>IgM levels was observed in both CVL and serum/plasma samples, the levels of total IgG or IgA in CVL were about 400-fold lower than those of the corresponding Ig in serum/plasma samples. Total IgM and IgG levels were found to be the lowest of all Ig isotypes present in CVL and RL, respectively.
HIV-1-Specific IgA and IgG Antibody Responses
The presence of HIV-1-specific antibodies of the IgA and IgG isotypes in serum/plasma, CVL, and RL of the HIV-1-infected women were determined by WB (Table 3). HIV-1-specific antibodies of the IgG isotype to the three ENV antigens (gp160, gp120, and gp41) were detected in the serum/plasma of all 63 HIV-1-infected women. In addition, bands of strong intensity representing HIV-1 p66, p55, p51, p31, p24, and p17 antigens were also prominent. HIV-1-specific antibodies to gp160, gp120, and gp41 of the IgA isotype were detected in 94%, 62%, and 86% of serum/plasma samples, respectively. However, the majority of the samples had fewer positive bands with much lower intensity (Fig. 1). HIV-1 ENV-specific IgG antibodies were readily detectable in CVL samples, though with lower intensity than in corresponding serum/plasma. 100%, 82%, and 73% of CVL samples were positive for IgA antibodies to gp160, gp120, and gp41, respectively. In spite of the 9-fold lower level of total IgG than IgA in RL samples (Table 2), the majority of RL samples contained IgG antibodies to HIV-1 ENV antigens. When present, IgA antibodies were mainly specific for gp160. These data demonstrated that IgG-binding antibodies to HIV-1 antigens were the dominant Ig isotype presented in the serum/plasma, CVL, and RL samples of HIV-1-infected women.
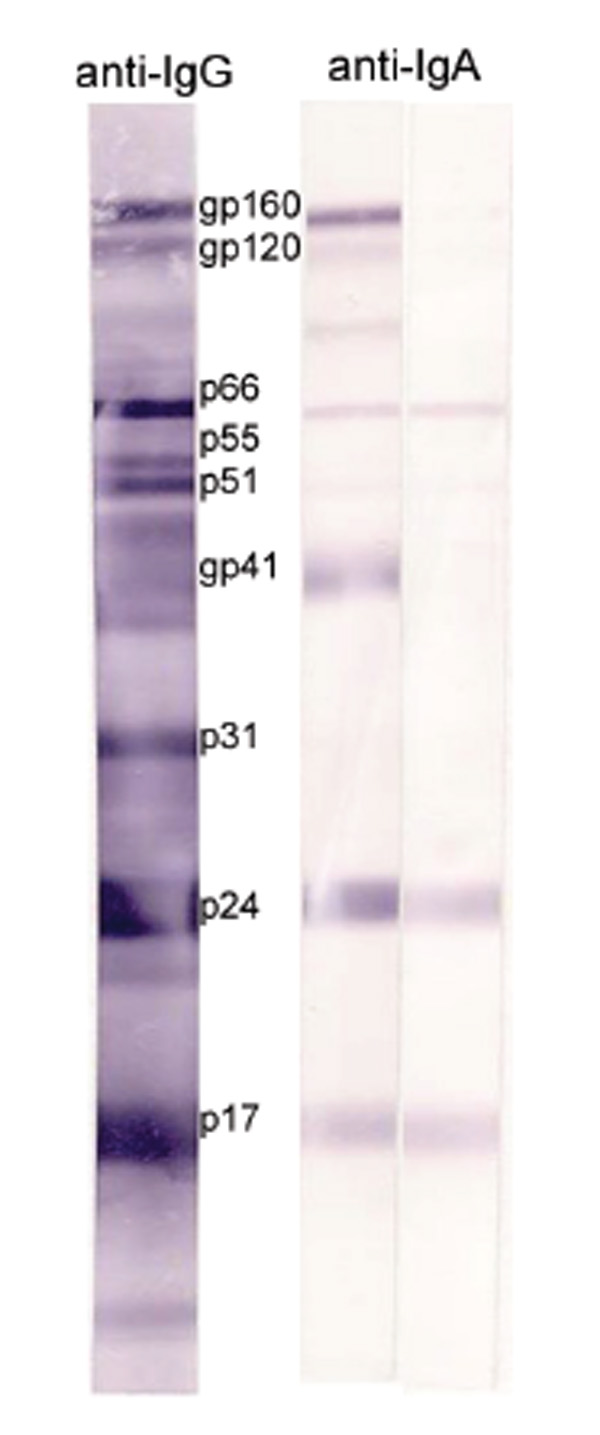 |
Fig. (1). HIV-1-specific IgG and IgA antibodies in serum/plasma samples determined by WB. Note the less intense bands and lower number of positive bands recognized by HIV-1-specific IgA antibodies. |
Ig in external secretions are not only produced locally but also derived from the circulation, and the relative contribution of the two origins to the Ig pools varies with Ig isotype and the type of secretion [21]. To determine the relationship of antibody responses to HIV-1 antigens in serum/plasma and the corresponding secretions, the patterns of HIV-1-specific antibodies of the IgG or IgA isotype were compared in parallel among 11 samples (Table 4). IgG antibodies to all 9 HIV-1 antigens detected in all serum/plasma were also found in 6 CVL and 4 RL samples. The other 5 CVL samples had IgG antibodies to 7 or 8 HIV-1 antigens. Of the remaining RL samples, 5 had IgG antibodies to 3-8 HIV-1 antigens and 2 had no detectable IgG antibodies to any HIV-1 antigens. IgA antibodies to 9 HIV-1 antigens were found in 4 serum/plasma samples, but none of the CVL or RL samples tested had IgA specific for all 9 HIV-1 proteins. The remaining 7 serum/plasma and all 11 CVL samples had IgA antibodies to 2-8 HIV-1 antigens. Of the 11 RL samples, 6 had IgA antibodies to 3-5 HIV-1 antigens, 4 had IgA antibodies to 1 HIV-1 antigen and 1 had no detectable HIV-1-specific IgA antibodies to any HIV-1 antigens. These data demonstrated that the similarity of external secretions to serum/plasma samples regarding the patterns of HIV-1-specific antibodies were greater for the IgG isotype than for IgA. Moreover, both IgG and IgA HIV-1-specific antibodies presented in CVL were closely related to those in serum/plasma, indicating that Ig from circulation might contribute to the Ig pool in CVL.
Neutralization Activity
Serum/plasma samples from 63 HIV-1-infected women were initially screened against three HIV-1 clade B viruses representing R5- (HIV-1SF162 and HIV-1YU2) and X4-tropic (HIV-1NL4.3) strains (Table 5). Three-fold dilutions of serum/plasma samples starting at either 1:20 or 1:30 were used (range of 1:20 to 1:43,740, and 1:30 to 1:21,870, respectively), and for a few samples the dilution ranged either 1:40 to 1:87,480, or 1:180 to 1:393,660. Of the 63 samples evaluated, 21 had ability to neutralize three viruses, 21 to two viruses (19 to HIV-1SF162 and HIV-1NL4.3, and 2 to HIV-1SF162 and HIV-1YU2), and 21 to only one (20 to HIV-1SF162, and 1 to HIV-1NL4.3). The median of IC50 titers to HIV-1SF162, HIV-1NL4.3, and HIV-1YU2 were 2,422, 265, and <30, respectively. Of the 42 samples exhibiting neutralizing antibody to at least two viruses, the 19 with relatively higher IC50 titers were selected and used to determine the contribution of IgA or IgG antibodies to neutralization activity (Table 7). Of the 19, 11 individuals’ corresponding CVL and RL samples were tested at 1:10 with two-fold dilutions. As expected, HIV-1-specific neutralization activity in CVL and RL samples was present at much lower levels than that in serum/plasma. The median of IC50 titers to HIV-1SF162, HIV-1NL4.3, and HIV-1YU2 were 23, 29, 19 among CVL samples and 28, 23, 19 among RL samples, respectively. The CVL from WARO and the RL from HUTA had the highest neutralization activity, and thus were selected to further determine the effect of Ig removal on virus neutralization (Table 9).
In addition, 5-6 samples that neutralized all three clade B viruses were selected and used to determine their ability to neutralize cross clade HIV-1. Experiments with clade C viruses, HIV-1TV1 and HIV-192BR025.09, were subsequently performed. In general, neutralization activity was detectable but at relatively low levels (Table 5).
Contribution of Ig to Neutralization Activity
To determine whether the inhibition of virus infectivity was indeed antibody-mediated, and to identify the Ig isotypes responsible for HIV-1 neutralization, depletion of IgG and/or IgA was performed on serum/plasma samples from 19 HIV-1-infected women, and CVL and RL samples from 3 of the 19 women. Neutralization capacity was then examined in parallel on samples before and after Ig depletion. As shown in Table 6, the removal of IgA and/or IgG from samples was effective and efficient. Complete removal of IgG (>99%) from serum/plasma and CVL samples was consistently achieved. In some cases, removal of IgG from RL was performed twice on the same sample to ensure that IgG was depleted. Depending on the distribution of IgA subclasses in different body fluids and individuals [45], total IgA depletion varied, since immobilized Jacalin binds to IgA1, but not to IgA2 according to the manufacturer’s instructions. Total IgA elimination from serum/plasma samples reached a maximum of 97%, while some IgA, probably IgA2, remained in IgA-depleted CVL and RL samples. Furthermore, the high variability in the proportion of depleted IgA in CVL was probably due to the fact that CVL samples were collected irrespective of the stage of the menstrual cycle. It is well known that the levels of IgA1 and IgA2 in cervical mucus are hormonally dependent and display remarkable variation before and after ovulation [46-49].
The reduction in IC50 to at least one clade B virus was observed after depleting IgA from all 19 serum/plasma samples evaluated (Table 7). Nevertheless, up to 3-fold reduction of IC50 to two clade B viruses was only seen in samples from HALE and WARO. Among 19 samples, DASH apparently had the strongest IgA responses to HIV-1 ENV antigens (Table 8) but IgA antibodies were only responsible for about half of the HIV-1SF162 neutralization, and had no effect on HIV-1NL4.3 neutralization. IgA antibodies in serum from HAAL, which also demonstrated stronger binding to HIV-1 ENV antigens, contributed predominantly to HIV-1SF162 neutralization, but little to HIV-1NL4.3, and not at all to HIV-1YU2 neutralization. In contrast, IgA antibodies in HALE contributed to more than half of neutralization activity to all three calde B viruses, although the capacity of IgA binding antibodies to HIV-1 ENV proteins was one of the weakest. These data suggested that the ability of IgA antibodies to bind HIV-1 ENV antigens or to neutralize HIV-1 viruses was not correlated. Removal of IgG resulted in a significant reduction in IC50 to at least two clade B viruses; in some samples (8/19) IC50 was reduced to under the detectable levels (lower than the starting dilution). Greater than 3-fold reduction in IC50 to HIV-1SF162, HIV-1NL4.3, or HIV-1YU2 was observed in 18, 14, and 9 samples, respectively, after IgG was removed. Taken together, these data demonstrated that both IgA and IgG antibodies in serum/plasma samples were responsible for inhibiting HIV-1 infectivity, but the neutralizing antibodies were present mainly in the IgG isotype.
As shown in Table 7, the removal of both IgG and IgA from 15/19 plasma/serum samples abolished neutralization activity to at least one clade B HIV-1. However, the inhibition of the three clade B HIV-1 infectivity remained at some levels in serum/plasma samples from 4 women (FOBI, MIUR, JOME, JODE) after both IgA and IgG were removed. Although HIV-1-infected women recruited for this study did not suppose to be on antiretroviral therapy (ART), these four individuals were reported later by the staff in 1917 clinic at UAB as ART users at the time of samples collection. Based on the pattern of IC50 to three clade B viruses determined in these women, two others were identified and confirmed as well. Therefore samples from 6 HIV-1-infected women were excluded from further analysis.
Contribution of Ig to neutralize clade C viruses was also examined with samples (HAAL, HUCA, and WARO) that were available in sufficient volumes for further Ig depletion. IC50 were reduced below detectable level after IgG removal, demonstrating that neutralizing antibodies to HIV-1TV1 and HIV-192BR025.09 were mainly IgG.
The neutralization activity detected in RL samples did not reflect antibody-mediated virus neutralization since IC50 titers to the three clade B viruses did not change in samples before and after Ig depletion (Table 9). However, in CVL samples the dominance of IgG over IgA on HIV-1 neutralization activity was observed. Removal of both IgA and IgG resulted in decreases of IC50 close to or below detectable levels. These data indicated that neutralization activity in CVL mainly resulted from HIV-1-specific neutralizing antibodies of the IgG isotype and to a lesser degree of the IgA isotype, and the contribution of other humoral innate factors was minimal.
DISCUSSION
Evaluation of humoral immune responses in external secretions in HIV-1-infected or vaccinated individuals is inevitably compromised by several important considerations. External secretions contain significantly lower levels of all Ig isotypes compared to serum, and the analysis is also complicated by their dilution with lavage fluids [36]. Furthermore, there is an enormous variation of Ig levels, particularly in CVL, influenced by hormonally-dependent changes during the menstrual cycle [46-49]. The endogenous or exogenous proteolytic enzymes may degrade Ig into fragments, causing the loss of secondary functions of antibodies such as the interference with multivalency of polymeric Ig, the damage of Fc region of Ig molecules essential in antibody-dependent cell-mediated cytotoxicity (ADCC) and antibody-dependent cell-mediated viral inhibitoin (ADCVI), and the compromised interactions with innate humoral factors [36]. Depending on the methods of collection, processing, and storage of external secretions, the variability in Ig levels of the same secretion may display up to 3 log differences [20, 50]. Therefore, the specific antibody activity should be always expressed with respect to the levels of total Ig [50]. Conventional assays, such as ELISA, used to measure IgA responses to HIV-1 have proven to yield sometimes unreliable, false-positive results [51]. Other tests, such as WB or enhanced chemiluminiscence (ECL)-WB, appear to be more sensitive, reliable and informative because the recognition between antibodies and HIV-1 antigens can be easily discerned [17, 18, 22, 38].
With these limitations and concerns for handling and examining external secretions, the results of our study confirmed earlier reports [17, 20], and clearly indicated that HIV-1-specific binding antibodies to HIV-1 proteins in serum/plasma, as well as CVL and RL are present predominantly in the IgG isotype. Corresponding IgA antibodies are found at considerably lower levels, and in external secretions, especially in RL, they are frequently absent. The determination of HIV-1-neutralizing antibodies in external secretions is complicated by the low levels of total Ig as compared to serum/plasma, and by the presence of innate humoral factors, such as secretory leukocyte protease inhibitor (SLPI) [23-25], human lactoferrin (hLf) [25-28], and others [29-30]. SLPI and hLf are the two indentified components responsible for HIV-1 inhibitory activity in different mucosal secretions and may interfere with the accurate measurements of neutralization mediated by antibodies. To confirm that the neutralization activity was indeed Ig-mediated, we selectively removed IgG antibodies by absorption on protein G and IgA1 on a specific lectin, Jacalin. In our previous studies, IgA antibodies specific to viruses, including HIV-1 [52] and the influenza virus [16, 53], were almost exclusively present in the IgA1 subclass. The importance of this approach was evident from the comparison of neutralization titers before and after the selective removal of IgG and IgA1. Although in the majority of serum/plasma samples such absorption led to a marked decrease in the neutralization titers, neutralization activity was detectable in six samples, apparently due to the presence of anti-viral activity. Such anti-HIV-1 inhibition in serum/plasma samples was due to the effect of ART. The corresponding CVL and RL samples from these subjects were not examined further in this study. Nevertheless, it became obvious that the depletion of IgG in CVL decreased the neutralization activity against HIV-1NL4.3, HIV-1SF162, and HIV-1YU2. The low titers of virus neutralization in RL were not substantially altered by removing IgG and/or IgA, suggesting that humoral innate factors with anti-viral activity, rather than Ig antibodies, were involved.
A parallel correlative comparison of HIV-1-specific antibodies of the IgG and IgA isotypes in CVL, RL and serum/plasma revealed that the CVL is more reminiscent of the corresponding serum/plasma antibodies than RL. This finding reflects the similarities in the Ig isotype distribution of HIV-1-specific antibodies present in serum/plasma and CVL, and confirmed the important contribution of Ig of circulatory origin to CVL fluid [14]. Furthermore, RL in which IgA of local origin is the dominant Ig isotype and IgG is found at extremely low levels [16-21, 51], and the well established fact that HIV-1-specific responses in all external secretions are associated with IgG [17-22], suggest that the immunization routes, protocols, and potential adjuvants that promote IgA responses to HIV-1 need to be explored.
CONCLUSIONS
The antibody responses to HIV-1 in serum/plasma and the genital and intestinal tracts are characterized by binding and neutralizing HIV-1-specific IgG and IgA antibodies in this study. Both HIV-1-binding and HIV-1-neutralizing antibodies of IgG isotype are displayed most frequently as HIV-1-specific Ig in serum/plasma and in the genital tract of HIV-1-infected women. In contrast, IgG binding antibodies found in RL do not inhibit HIV (HIV-1) infection.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
ACKNOWLEDGEMENTS
Funding for this project was provided by the US PHS program projects 5 P01 AI085027, grant DK064400, grant AI083613, and grant AI093151. The authors thank Patricia Grayson and Julie Decker for their kind helps.







