Validation of Microcapillary Flow Cytometry for Community-Based CD4+ T Lymphocyte Enumeration in Remote Burkina Faso
Abstract
Background
CD4+ T lymphocyte enumeration plays a critical role in the initiation and monitoring of HIV-infected patients on antiretroviral therapy. There is an urgent need for low-cost CD4+ enumeration technologies, particularly for use in dry, dusty climates characteristic of many small cities in Sub-Saharan Africa
Design
Cross-sectional study
Methods
Blood samples from 98 HIV-infected patients followed in a community HIV clinic in Ouahigouya, Burkina Faso were obtained for routine CD4+ T lymphocyte count monitoring. The blood samples were divided into two aliquots, on which parallel CD4+ measurements were performed using microcapillary (Guava EasyCD4) and dedicated (Becton Dickinson FACSCount) CD4+ enumeration systems. Spearman rank correlation coefficient was calculated, and the sensitivity, specificity and positive predictive value (PPV) for EasyCD4 <200 cells/µL were determined compared to the reference standard FACSCount CD4 <200 cells/µL
Results
Mean CD4 counts for the EasyCD4 and FACSCount were 313.75 cells/µL and 303.47 cells/µL, respectively. The Spearman rank correlation coefficient was 0.92 (p<0.001). Median values using EasyCD4 were higher than those with the FACSCount (p=0.004). For a CD4<350 cells/uL, sensitivity of the EasyCD4 was 93.9% (95%CI 85.2-98.3%), specificity was 90.6% (95% CI 75.0-98.0%), and PPV was 95.4% (95%CI 87.1-99.0%)
Conclusion
Use of the EasyCD4 system was feasible and highly accurate in the harsh conditions of this remote city in Sub-Saharan Africa, demonstrating acceptable sensitivity and specificity compared to a standard operating system. Microcapillary flow cytometry offers a cost-effective alternative for community-based, point-of-care CD4+ testing and could play a substantial role in scaling up HIV care in remote, resource-limited settings
INTRODUCTION
The roll out of antiretroviral therapy (ART) in developing countries has led to a focus on the creation of sustainable healthcare systems through improved medical infrastructure. In this regard, there has been increasing recognition of the importance of community-based care and treatment as a means to increase access to ART for people living with HIV/AIDS in severely constrained settings
In this era of increased affordability and availability of ART, there is a critical need for low cost, high throughput, point-of-care monitoring of patients on antiretroviral therapy. When evaluating the cost-effectiveness of various antiretroviral treatment and monitoring strategies (e.g. using CD4+ T lymphocyte enumeration, total lymphocyte count or HIV plasma viral load), patient monitoring using CD4+ T lymphocyte enumeration has been found to be the most cost-effective strategy in resource-limited settings [1]. In this regard, the WHO recommends that the absolute number of CD4+ T lymphocytes be used as a guide to initiate ART [2], to initiate prophylaxis against opportunistic infections, and to discontinue these prophylactic medications [3]. Importantly, as HIV viral load assays are not widely available in the developing world, it is the absolute number of CD4+ cells that is frequently used to assess an individual’s response to ART [2]. In order to improve access to HIV/AIDS care and treatment in resource-poor countries, there is an urgent need for better technologies to measure the absolute number of CD4+ T lymphocytes
Flow cytometry is the gold standard method used for CD4+ T lymphocyte enumeration. This technology measures the intensity of fluorescence as cells labeled with fluorescent antibodies pass through a laser beam. Conventional flow cytometry is open platform (i.e., not dedicated), meaning that reagents from various suppliers may be used and that the technology can potentially be used to perform assays other than CD4+ cell enumeration. Other advantages include high throughput and that some conventional systems do not require red blood cell lysis, which reduces both time and cost. The major disadvantages of the conventional systems are the need for a separate hematology analyzer in the dual platform systems, the complexity of the equipment (often requiring intensive training for several months), as well as the cost of the machines themselves [4]. Alternatively, dedicated flow cytometers (e.g., FACSCount; Becton Dickinson Immunocytometry, San Jose, California) are used for the single purpose of CD4+ T lymphocyte enumeration. Advantages of the dedicated systems are a lower cost of the machines, lack of a need for a separate hematology analyzer and a decreased potential for human error given the minimal number of steps requiring operator intervention. The disadvantages of this technology include a restriction on reagents to only those produced by the manufacturer and that the machines cannot be used to perform types of assays beyond the absolute CD4+ cell count [4]
Microcapillary flow cytometry (e.g., EasyCD4; Guava Technologies, Hayward, California) uses a unique technique compared to conventional or dedicated flow cytometry. One important difference is that microcapillary flow cytometry does not require the use of sheath fluid to move cells through the laser beam; instead, cells pass single-file through a microcapillary filament. The lack of sheath fluid not only reduces cost but it also substantially reduces the amount of bio-hazardous waste generated, which is an important consideration in developing countries. The machine is also compact in size and therefore it potentially could be transported to rural clinic sites. Other advantages of microcapillary flow cytometry include the use of a low blood volume per sample, relatively easy-to-learn technology with minimal training required, low inter-laboratory variability presumably due to simplistic design and the potential to perform different types of assays (i.e., in addition to CD4+ cell counts). The reagents are also less expensive, and the Guava business model allows the use of reagents produced by a variety of manufacturers. Lastly, the Guava EasyCD4 also requires minimal maintenance, as the only components handled by laboratory personnel are the power switch, the sample loader and the waste vial [4]. Probably the most important advantage of microcapillary flow cytometry in developing countries is its overall low cost compared to conventional or dedicated flow cytometric systems [5]
The Guava EasyCD4 has been evaluated in comparison to standard flow cytometry, specifically the BD FACSCount, for CD4+ T cell enumeration in HIV-infected persons in large urban settings in Uganda, India and Thailand. Correlation between the two systems was found to be 0.96 in Kampala, Uganda [6], 0.994 in Vellore, India [6], and 0.97 in Bangkok, Thailand [7]. In Chennai, India, Balakrishnan, et al used both systems to test 110 samples from HIV-infected persons and found that the EasyCD4 assay had a sensitivity of 95% and a specificity of 100% for the identification of CD4+ T cell counts <200 cells/µL [8] The EasyCD4 has also been compared to conventional flow cytometry systems, with correlations of 0.938 in comparison with the FACSORT in India [9] and 0.97 in comparison with the FACSCalibur/TruCOUNT method in Thailand [7]. In the United States, the EasyCD4 had comparable CD4+ T lymphocyte count estimates when compared to standard flow cytometry [5]. Notably, all of the above-mentioned validation studies were performed in relatively developed urban medical centers
The objective of this study was to compare the performance of the Guava EasyCD4 to the Becton Dickinson FACSCount for community-based CD4+ T lymphocyte monitoring of HIV-infected patients in Burkina Faso. Burkina Faso is a country of over 14 million people with an adult HIV prevalence of 1.6% in 2007 [10]. UNAIDS reports that, at the end of 2007, 130,000 Burkinabé were living with HIV, and 61,000 of those infected were women aged 15 years or older [10]. The country is located within the Sahel, the horizontal strip of Africa laying between the Sahara Desert in the north and the more fertile regions in the south. Its tropical climate has two distinct seasons: a wet season from May to September and a prolonged dry season that is marked by the harmattan, the hot, dusty wind from the Sahara. This study was performed during the dry season in the northern city of Ouahigouya, a relatively small city of approximately 65,000 inhabitants. In addition to comparing the performance of the Guava EasyCD4 to the FACSCount, a second objective of the study was to assess the feasibility of using microcapillary flow cytometry in a relatively undeveloped city that has a dry, dusty climate representative of many small cities in Sub-Saharan Africa. Similar to many remote laboratories in Sub-Saharan Africa, the community laboratory where our study was performed is unlike the laboratories in the large cities and academic medical centers where the EasyCD4 system has been evaluated in prior studies. Specifically, the windows in the Ouahigouya laboratory are not enclosed with glass; instead, metal shutters are the only barrier separating the laboratory from the outside air, and the shutters do not effectively prevent entry of external dust into the laboratory. Secondly, there is no climate control, and the laboratory is ventilated only by ceiling and floor fans. Lastly, although the laboratory is powered by electricity, thereby allowing use of medical equipment and refrigeration of the system reagents, the frequent power outages are problematic as there is no back-up generator available for use
METHODS
Consecutive whole blood samples from 98 HIV-infected patients followed in a community HIV clinic in Ouahigouya were obtained for routine CD4+ T lymphocyte count monitoring. The community clinic, Appui Moral, Material et Intellectuel à l’Enfant (AMMIE), is one of the six AIDS Empowerment and Treatment International (AIDSETI) associations in Burkina Faso. AIDSETI is a network of community-based clinics that has been funded by the World Bank Treatment Acceleration Program (TAP) [11]. The unique model of AIDSETI has been described previously [12]. HIV was diagnosed using the Bioline HIV 1-2 assay (Standard Diagnostics, Inc.). Patients gave informed consent to receive medical care at AMMIE, which included intermittent monitoring of CD4+ cell counts. Laboratory results were unlinked from patient identifiers before analysis. The study protocol was approved by the Institutional Review Board at Stanford University School of Medicine
Blood samples were obtained from January to March 2006. Each blood sample was divided into two aliquots, on which parallel CD4+ count measurement was performed using the EasyCD4 and the FACSCount systems. One technician performed testing on the EasyCD4, and two technicians performed testing on the FACSCount. The technicians were blinded to the test results on the other system. Testing was performed using the EasyCD4 and the FACSCount as directed by the respective manufacturer’s instructions [13, 14]. Reagents used were from the same lot for all tests performed on the EasyCD4. All of the samples were processed by both systems within 24 hours of the time that the blood was obtained
The mean CD4+ cell counts as estimated by the EasyCD4 and by the FACSCount were determined. The Spearman rank correlation coefficient (r) was calculated, and the agreement between the EasyCD4 and the FACSCount was evaluated by Bland-Altman analysis [15]. The Wilcoxon Sign test was used to assess the differences in the median CD4+ cell counts as determined by the two systems. All statistical tests were performed using SPSS statistical software (SPSS, Inc., Chicago, Illinois, USA)
The samples were divided into three groups for further analysis: samples with FACSCount CD4+ count <200 cells/µL, FACSCount CD4+ count 200-350 cells/µL and FACSCount CD4+ count >350 cells/µL. The mean and median CD4+ cell count estimations as determined by the EasyCD4 were calculated for each of the three groups, as were individual Spearman rank correlation coefficients. The sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) for EasyCD4 <350 cells/uL were determined compared to the standard FACSCount CD4 <350 cells/uL. The CD4 cell count of 350 cells/uL was chosen as the breakpoint for data analysis given its clinical relevance, as WHO recommends initiating ART when the CD4+ T lymphocyte count reaches this level [2]. Sensitivity and specificity were also calculated for correct identification of cell counts between 200 and 350 cells/uL, to assess the characteristics of the EasyCD4 system when counting cells in patients with critically low CD4+ cell numbers
RESULTS
Of the 98 subjects, 23.5% were men and 76.5% were women. The mean age of the study participants was 36.7 years. The mean age of the male subjects was 41.6 years (range 16-70 years) and the mean age of the female subjects was 35.2 years (range 8-64 years). All of the subjects were infected with HIV-1; none were infected with HIV-2. 29.6% of the subjects were receiving ART at the time that the blood samples were obtained
The mean CD4+ T lymphocyte count for the EasyCD4 and FACSCount was 313.75 cells/µL (S.D.=196) and 303.47 cells/µL (S.D.=229), respectively. Fig. (1) depicts the correlation plot of CD4+ cell counts using the two systems, with a calculated Spearman rank correlation coefficient (r) of 0.92 (p<0.001). Fig. (2) displays the Bland-Altman analysis, which illustrates that the CD4+ counts calculated by the two systems were the most similar and had the least variability in the lower CD4+ count ranges, specifically in the range of CD4+ <350 cells/µL. The differences in the CD4+ counts as measured by the two systems increased as the absolute CD4+ count increased, especially for CD4+ counts above 350 cells/µL
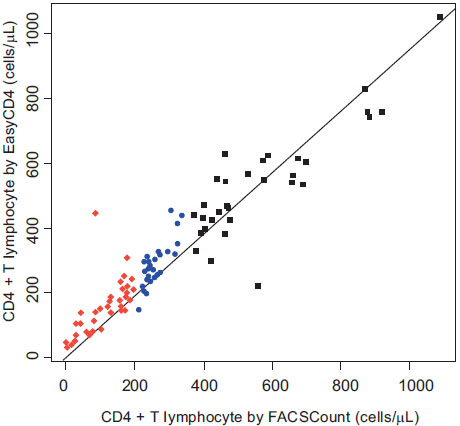
Correlation Analysis of the EasyCD4 assay and FACSCount for cell counts (cells/µL), divided into CD4+ T lymphocyte count by FACSCount <200 cells/µL (red diamonds), 200-350 cells/µL (blue circles), and >350 cells/µL (black squares). Spearman rank correlation coefficient = 0.92 (P<0.001). The solid line represents the regression line. Mean CD4 counts for the EasyCD4 and FACSCount systems were 313.75 cells/µL and 303.47 cells/µL, respectively
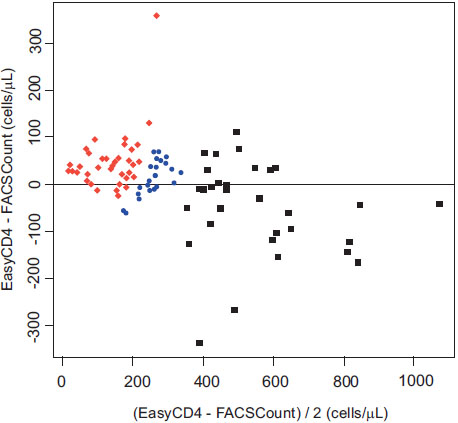
Bland-Altman Plot (n=98, HIV-infected individual whole blood samples) to establish agreement between the EasyCD4 assay and FACSCount for CD4+ T lymphocyte enumeration. CD4+ T lymphocyte count by FACSCount is divided into <200 cells/µL (red diamonds), 200-350 cells/µL (blue circles), and >350 cells/µL (black squares). The solid horizontal line represents the mean difference between the two systems
The CD4+ T lymphocyte count estimates by the EasyCD4 were frequently higher than those obtained with the FACSCount (p=0.006). Specifically, 62 EasyCD4 measurements were higher than the FACSCount, 35 EasyCD4 estimates were lower than the FACSCount, and 1 EasyCD4 result was identical to that of the FACSCount. The EasyCD4 measurements were generally higher than the FACSCount measurements for CD4+ counts <200 cells/µL and 200-350 cells/µL (mean differences of +45.5 and +25.1 cells/µL, respectively), and the EasyCD4 measurements were lower than the FACSCount measurements for CD4+ count >350 cells/µL (mean difference of –45.3 cells/µL)
The EasyCD4 had a sensitivity of 93.9% (95%CI 85.2-98.3%) and specificity of 90.6% (95%CI 75.0-98.0%) to identify CD4+ T lymphocytes <350 cells/uL when compared to the FACSCount. The PPV for the Easy CD4 in this cell range was 95.4% (95%CI 87.1-99.0%) and NPV was 87.9% (95%CI 71.8-96.6%). The sensitivity and specificity for correct identification of CD4+ cells within the 200-350 cells/uL range were 74.1% (95%CI 53.7-88.9%) and 81.7% (95%CI 70.7-89.9%), respectively
A total of 39 samples had a CD4+ count <200 cells/µL as measured by the FACSCount; the EasyCD4 mean for this group was 157 cells/µL (S.D.=81), the EasyCD4 median was 155 cells/µL (range 31-445) and the Spearman correlation coefficient was 0.823. For the 27 samples that had a CD4+ count 200-350 cells/µL as measured by the FACSCount, the EasyCD4 mean was 283 cells/µL (S.D.=74), the EasyCD4 median was 271 cells/µL (range 144-454) and the Spearman correlation coefficient was 0.771. A total of 32 samples had a CD4+ count >350 cells/µL as measured by the FACSCount; for these samples, the EasyCD4 mean was 538 cells/µL (S.D.=169), the EasyCD4 median was 537 cells/µL (range 220-1050) and the Spearman correlation coefficient was 0.709
DISCUSSION
The aim of this study was to demonstrate the feasibility of using the Guava EasyCD4 system in a small, remote city in Sub-Saharan Africa as well as to compare its performance to a widely used technology, the Becton Dickinson FACSCount. These results indicate that the use of microcapillary-based technology is feasible for community-based HIV/AIDS healthcare in this small city in Sub-Saharan Africa, and specifically using a laboratory that lacks a glass window barrier to the external dust, climate control, and a stable source of electricity. The results are encouraging, as there was excellent correlation between the two systems for CD4+ T lymphocyte enumeration in this relatively remote setting in Burkina Faso. Specifically, there was a correlation of 92% between the EasyCD4 and the FACSCount by Spearman rank statistics, which was statistically significant (p<0.001). Based on these results, it is apparent that the EasyCD4 is a cost-effective alternative for community-based, point-of-care CD4+ testing, and that it could play a substantial role in scaling up HIV care in remote, resource-limited settings
As is illustrated in the two figures, the correlation between the two systems was highest in the CD4+ T lymphocyte range <350 cells/uL. Notably, because the WHO HIV treatment guidelines recommend that ART be initiated when the CD4+ cell count reaches 350 cells/uL [2], a high correlation between the two systems is most critical at this range of CD4+ cells. The performance of the EasyCD4 was more variable as the CD4+ cell count increased >350 cells/µL, which is consistent with several other studies evaluating the performance of the EasyCD4 assay [5, 7, 8]
Using the Guava EasyCD4 system, the CD4+ T lymphocyte counts were slightly higher compared to the FACSCount for CD4+ counts <350 cells/uL, but were generally lower than the FACSCount for CD4+ counts >350 cells/uL. The observation that the EasyCD4 system may give higher cell count estimates than standard flow cytometry has been reported in prior studies [5-8], and clinicians should be aware of the tendency of the EasyCD4 to produce higher CD4+ cell count estimates in some cell count ranges. This finding could have clinical implications: if the EasyCD4 cell counts <350 cells/uL are indeed an overestimation of the actual CD4+ cell count, an overestimation could potentially result in an erroneous delay in the initiation of ART
Importantly, this study has several limitations. First, in order to validate a technology for use in developing countries, it is important to assess the reproducibility of the results after waiting for a period of time after specimen collection. This is especially important in resource-limited settings where there is frequently a delay in the time to testing from the time that the blood samples are obtained. Secondly, we did not evaluate for potential operator bias; ideally, we would have studied the variation in test results for each blood sample when several different laboratory personnel operated the machine. A third limitation is that we did not perform the validation in HIV-negative healthy controls; therefore, it is unclear if HIV-infected status has an impact on the accuracy or correlation of the two technologies. Similarly, as no HIV-2 infected subjects were included in this study, it is also unclear if the type of HIV has an impact on CD4+ T lymphocyte enumeration. It may also have been useful to assess the correlation between the two machines for CD8+ T lymphocyte counts in addition to CD4+ T lymphocyte counts
There are several notable challenges of using low-cost technologies for CD4+ T lymphocyte enumeration in devel-oping countries. Issues pertaining to quality assurance and quality control are in the forefront, as it is critical to ensure that samples are processed correctly and to ensure that test results are comparable between individual laboratories. Secondly, access to routine technological maintenance can be difficult, especially in remote parts of developing countries. Excessive heat and dust are frequently problematic, particularly in Sub-Saharan countries with dry and sandy conditions; the dust may interfere especially with technical components such as lasers. Unreliable sources of electricity are a common problem, not only creating difficulties with using the machines themselves but also for maintaining the required refrigeration of the reagents. The reagents may have a relatively short half-life and therefore require frequent replenishment of supplies, which can be challenging in remote areas
Low-cost diagnostics are intrinsic to effective HIV/AIDS health service delivery. These results demonstrating the feasibility of microcapillary flow cytometry are promising, but novel technologies are urgently needed. Developments in low-cost diagnostic test methods for the monitoring of HIV-infected individuals are underway. Transfix is a blood stabilizing compound that permits accurate CD4+ T lymphocyte enumeration more than 48 hours after blood has been obtained, and it has been studied for use with the Guava system2. There is also a recently developed microchip that, using the principle of microfluidics, appears promising as a simple method for CD4+ T lymphocyte enumeration [16]. Further research into patterns of utilization and cost-benefit analyses need to be performed in order to define effective systems for monitoring patients with HIV/AIDS
CONFLICTS OF INTEREST
None of the authors have a reported financial, consultant, institutional or other conflict of interest in the publication of this manuscript
NOTES
1 Josefowicz SZ, Louzao R, Lam L, etal. Five-site evaluation of the Guava EasyCD4 assay for the enumeration of human CD4+ T cells [Abstract U-138]. Presented at: th Conference on Retroviruses and Opportunistic Infections; 2005; Boston.
2 2Mergia A, Elad K, Sharp M, Fishwild D, O’Connell B, Bredt B, etal.Performance of aged, Transfix-treated blood in the Guava EasyCD4 and EasyCD8 assays [Abstract 739]. Presented at: 12th Conference on Retroviruses and Opportunistic Infections; 2005; Boston
ACKNOWLEDGEMENTS
We acknowledge the participants, as well as the laboratory staff at AMMIE and at Centre Hospitalier Régional in Ouahigouya for their assistance. We especially acknowledge Dr. Ibrahim Sieba of Centre Hospitalier Régional, Ouahigouya, who assisted with sample processing using the FACSCount. We also would like to recognize our sources of financial support, namely the National Institutes of Health (NIH) training grant #AI052073 (Renault), an unrestricted grant from The Gilead Foundation, and The World Bank Treatment Acceleration Program (TAP)


