Assessment of the Effect of HAART on Renal Function of HIV Patients Attending the Bamenda Regional Hospital, Cameroon
Abstract
Background:
Management of HIV involves a life-long administration of a cocktail of antiretroviral drugs, some of which have been known for their nephrotoxicity. Despite the increasing access to this combination therapy, Highly Active Antiretroviral Therapy (HAART) information on its renal effect is still scarce and contradictory. The aim of this study was to assess the effect of HAART on the renal function of HIV-infected patients attending the Bamenda Regional Hospital, Cameroon.
Methods:
This was a comparative hospital-based cross-sectional study involving HIV positive and negative individuals who visited the Day clinic of the Bamenda Regional Hospital during the study period. Spectrophotometry was used to quantify the renal markers. Glomerular Filtration Rate was determined by the 24 hours creatinine clearance method. Blood urea nitrogen was calculated from serum urea concentrations. Renal impairment was then classified according to the National Kidney Foundation clinical practice guideline. Data were analysed on SPSS version 21 using Student t-test, ANOVA, and Pearson’s correlation. The level of significance was set at p<0.05.
Results:
A total of 201 participants were enrolled in this study, of which 144(71.6%) were females. Their ages ranged between 22 to 60 years with a mean age of 37.4 ± 9.6 years. The participants were divided into 3 study groups; HIV negative, HAART-naïve and the HAART experienced groups. The HAART experienced group had a significantly higher mean BUN and BUN-Creatinine ratio (p= 0.001 and 0.003 respectively) as well as the least creatinine clearance (p= 0.017) when compared to the other groups meanwhile the HAART-naive group had a significantly higher mean urine protein (p= 0.026) when compared to the other two categories. There was no association between renal dysfunction and the HAART regimen as well as adherence to treatment.
Conclusion:
This study demonstrated that though the participants on HAART had decreased renal function, the mean Creatinine clearance was not statistically different from that of the participants not yet on HAART. this is indicative that the decreased renal function could be as a result of the devastating effect of HIV. It further demonstrates no association between decreased renal function to the type of HAART regimen used, duration on HAART as well as the patient’s adherence to treatment.
1. BACKGROUND
Despite the interventions put in place by the world Health Organization (WHO), Human Immunodeficiency Virus (HIV) still remains a major public health problem. Though there has been a decrease in new diagnoses of HIV infection from 2.8 million in 2000 to about 1.8 million in 2017 [1], there are an increasing number of people living with HIV/AIDS (PLWHIV) placed on antiretroviral therapy (ART), with reports of 8 million people in 2010 to about 21.7 million people in 2017, worldwide [1]. It is noteworthy that there has been a decrease in the number of AIDS-related deaths from 1.4 million in 2010 to 940,000 in 2017 [1].
In Cameroon, 28,000 new HIV infected cases were diagnosed in 2017 and 510,000 people were living with HIV [2]. The prevalence of HIV amongst the population age 15 to 49 years in Cameroon in 2017 was estimated to be 3.7% [3]. Within Cameroon, the highest HIV prevalence was reported in the South (6.3%), East (5.9%), Centre (5.8%) and North West (5.1%) regions and the lowest in the Far North (1.5%) and North (1.6%) regions [3].
The association between HIV and renal disease was first reported in 1984 by Rao and colleagues, on a series of HIV-1 seropositive patients who developed renal syndrome characterized by progressive renal failure and proteinuria [4]. Since the introduction of Highly Active Antiretroviral Therapy (HAART), a combination of at least three antiretroviral drugs from two different drug classes in the management of HIV/AIDS in the mid-1990s, there has been a drastic drop in the number of AIDS-related deaths [5, 6]. The use of these antiretroviral drugs has been reported to be associated with a number of adverse effects including those affecting the kidney [7]. Problems with kidney function in HIV infected patients may be due to medications or HIV itself [8]. The kidney plays a major role in the metabolism and excretion of most drugs including antiretroviral drugs and other nephrotoxic drugs [9]. Given the high rate of blood flow through the proximal tubule and consequently the high level of toxins it has to process, this portion of the nephron is at particular risk of developing drug-related damage [9]. Thus, HAART which involves a cocktail of antiretroviral drugs may increase the risk of kidney damage which could either be due to drug-drug interaction or the nephrotoxicity of the drugs themselves [9].
Despite the high prevalence of renal dysfunction reported in many sub-saharan African countries [10-15], data on renal function after HAART initiation is scarce and contradictory. Contrary to reports on the nephrotoxicity of antiretroviral drugs and the association of HAART to chronic deseases like diabetes and hypertention which are the primary causes of kidney damage [15, 16], some studies in sub-saharan Africa have reported HAART to decrease the rate of renal damage [17-20]. Although current guidelines recommend the determination of renal function prior to HAART initiation, this is often not performed due to resource constraints in many areas in sub-Saharan Africa including Cameroon [21].
Our study objective was to determine if HAART further worsens or reverses the renal damages caused by HIV.
2. METHODS
2.1. Study Site
This study was conducted at the Day clinic of the Bamenda Regional Hospital located in Bamenda, the capital of the North West Region of Cameroon. Bamenda regional hospital is the leading tertiary care hospital in the region, providing care and treatment to over 5000 PLWHIV as in 2016. The North West Region was ranked fourth (5.1%) in the CAMPHIA 2017 HIV prevalence ranking in Cameroon [3].
2.2. Study Design and Setting
This was a comparative hospital-based cross-sectional study involving both HIV positive and negative adults within the age group of 21 to 60 years. Study participants were recruited at the Day clinic of the Bamenda Regional Hospital. Recruitment of participants took a span of 4 months from March to June 2016.
Study population: Participants were recruited from HIV-infected patients who came to the centre for drug refill or routine check-up during the study period or healthy individuals who came to the clinic for voluntary HIV counseling and testing (VCT). The study population was recruited under 3 study categories: participants who were HIV negative, HIV infected participants not yet on HAART (HAART-naïve group) and HIV infected participants on HAART (HAART experienced group).
2.3. Inclusion and Exclusion Criteria
Adults, males and females aged between 21 to 60 years who voluntarily accepted to take part in this study and signed the written consent form, were recruited into the study. This is because according to the Cameroon code for the classification of adults, adulthood begins at the age of 21 years. There are reports of a higher probability of having reduced renal function among adults older than 60 years [22]. Therefore in an effort to reduce renal effect due to age, only participants younger than 60years were considered in this study. Participants who had a blood pressure >140/90mmHg and/or fasting blood sugar >120mg/dl were excluded. Participants who were obese, suffering from urinary track infection or any other acute disease, had history of kidney transplant or dialysis, or those with known kidney, liver or heart diseases as well as pregnant women were also excluded from the study. Participants on long-term medications other than HAART were not included.
2.4. Sample Size Determination
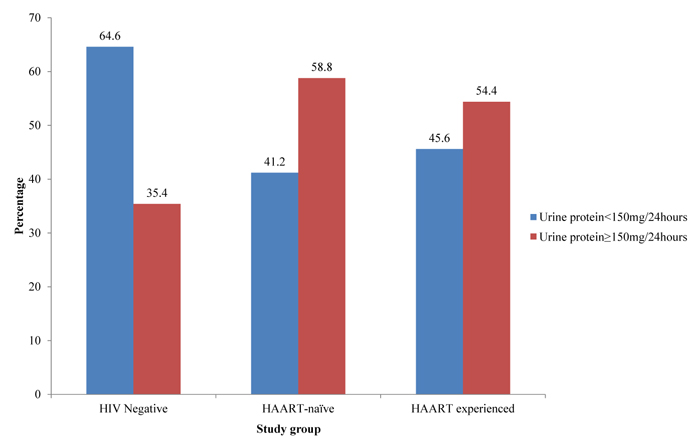
Considering prevalence of renal dysfunction among HIV patients on HAART of 14.3% [16], at a 5% error margin, the following statistical formula was used to determine the sample size for this study.
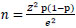 Where n =sample size, Z= constant= 1.96, p = prevalence= 14.3%, e= error margin.
Where n =sample size, Z= constant= 1.96, p = prevalence= 14.3%, e= error margin.
2.5. Data Collection and Measurements
2.5.1. Administration of Questionnaires
Data on the participants’ socio-demographic characteristics and medical history were collected using a structured questionnaire. Demographic data included sex, age, residence, level of education, alcohol consumption, residence, marital and employment status. Participants resident in the cities or towns were considered ‘Urban’ and from the villages considered as ‘rural. Participants with an education level above secondary (College) were considered under tertiary. Participants were considered as consumers of alcohol if they had consumed at least 1glass of alcohol per week within the past 5 years. Participants were considered as adherent to treatment if they did not miss more than two doses per month Participants’ hospital records were also reviewed to obtain information on the latest CD4 count (not greater than 2months old), the type of drug regimen and the duration on treatment.
2.5.2. Sample Collection
About 2 mls of venous blood sample was collected from each participant into a labeled dry tube using a vacutainer needle. These blood samples were allowed to clot at room temperature and centrifuged at 3000rpm for 5mins to obtain sera. The sera were aliqouted into labeled Eppendorf tubes and stored at -20oC and were later analyzed in batches for Urea and Creatinine concentrations .
The participants were requested to provide about 50 ml on-spot urine in a small, leak-proof container which was used for urinalysis for the screening of urinary tract infection (Positive nitrite and leucocytes). Recruited participants were then given corked graduated 5L containers containing a small amount of preservative for a 24 hours urine sample after the collection process has been explained to them in detail. The urine samples were brought to the laboratory immediately after the collection, where the volumes were measured and about 2ml samples pipetted into dry tubes, and stored at 2-8oC. The urine samples were then analyzed the next day for Urine creatinine and protein concentrations.
2.6. Laboratory Procedures
Serum and Urine Creatinine levels were analyzed spectrophotometrically with an ERMA BIOCHEMICAL ANALYZER (ERMA INC. Tokyo Japan, Model AE-600N) following the manufacture’s instructions. Urea and urine proteins were measured using a 3000 EVOLUTION spectrophotometer. Serum and urine creatinine concentrations were then used to calculate Creatinine clearance (Crcl) while serum urea and creatinine were used to calculate blood urea nitrogen (BUN) and BUN-creatinine ratio.
Renal impairment was classified according to the National Kidney Foundation clinical practice guideline based on the GFR determined by the Crcl method. Accordingly, estimated creatinine clearance values ≥ 90ml/min/1.73m2, 60-89 ml/min/1.73 m2, 30-59 ml/min/1.73 m2, 15-29 ml/min/1.73 m2 and < 15ml/min/1.73 m2 were interpreted as normal, mild, moderate, severe impairment and the most severe as kidney failure [23]. Renal dysfunction was defined as Crcl <60ml/min/1.73m2. Reference ranges for renal function tests were set as follows; Crcl >90ml/min/1.73m2, BUN 10-20mg/dl, BUN-Creatinine ratio 10-20, and urine protein <150mg/24hrs.
2.7. Data Management and Statistical Analysis
Codes were assigned to each participant so as to observe strict confidentiality. The results were entered in a secured log book. The questionnaires and logbooks were treated with strict confidentiality. Data was then entered into Microsoft excel version 10, cross-checked for errors and then transferred into the statistical package for social sciences (SPSS) version 21 for analysis. The differences between group means were compared using the analysis of variance (ANOVA) or student t-test. Fisher’s Least Significant difference multiple comparison was used for post hoc analysis for significant ANOVA comparison. Categorical data were compared using chi-square test. Pearson’s correlation was used to correlate variables. Statistical significance was set at p < 0.05.
3. RESULTS
3.1. Demographic Characteristics of Participants
A total of 206 adults visiting the Day Clinic of the Bamenda Regional Hospital were enrolled into the study. Three participants were excluded due to a blood pressure of >140/90mmHg and after laboratory analysis, two more patients were excluded for positive Nitrite and Leucocyte in their urine samples leaving behind a total number of 201 participants for the data analysis. The study population was sub-divided into three study groups; 65(32.3%) participants were HIV negative, 68(33.8%) were HIV positive patients not yet on HAART (HAART-naïve group) and 68(33.8%) were HIV patients on HAART (HAART experienced group). There were 57(28.4%) males and 144 (71.6%) females. The participants had ages ranging from 22 to 60 years with a mean of 37.4±9.6 years. The majority of the participants, 64(31.8%) were within the age group of 41 to 50 years. The HAART experienced group had the highest mean age, BMI, SBP/DBP and FBS among the study categories.
Table 1 summarises the sociodemographic characteristics of the study population.
Fig. (1) describes the distribution of participants on the various HAART regimens. A majority of the participants on HAART (82.4%) were on tenofovir-based regimen and had a mean duration on treatment of 6.95±3.74years.
| Socio-demographic characteristics | Study groups | ||||
|---|---|---|---|---|---|
| HAART-naïve n=68 |
HAART experienced n=68 |
HIV negative n=65 |
Total N=201 |
||
| Number (%) | Number (%) | Number (%) | Number (%) | ||
| Sex | Male | 22(32.4) | 14(20.6) | 21(32.3) | 57(28.4) |
| Female | 46(67.6) | 54(79.4) | 44(67.7) | 144(71.6) | |
| Age | 21 -30 | 20(29.4) | 6(8.80 | 33(50.8) | 59(29.4) |
| 31 -40 | 23(33.8) | 19(27.9) | 18(27.7) | 60(29.8) | |
| 41 -50 | 20(29.4) | 33(48.5) | 11(16.9) | 64(31.8) | |
| >50 | 5(7.4) | 10(14.7) | 3(4.6) | 18(9.0) | |
| Marital status | Married | 36(52.9) | 34(50.0) | 35(53.8) | 105(52.2) |
| Single | 22(32.4) | 20(29.4) | 30(46.2) | 72(35.8) | |
| Divorced | 1(1.5) | 3(4.4) | 0(0.0) | 4(2.0) | |
| Widowed | 9(13.2) | 11(16.2) | 0(0.0) | 20(10.0) | |
| Employment status | Employee | 14(20.6) | 16(23.5) | 22(33.8) | 52(25.9) |
| Self Employed | 38(55.9) | 34(50.0) | 24(36.9) | 96(47.7) | |
| Student | 6(8.8) | 1(1.5) | 12(18.5) | 19(9.4) | |
| Unemployed | 10(14.7) | 17(25.0) | 7(10.8) | 34(17.0) | |
| Level of education | Primary | 30(44.1) | 41(60.3) | 16(24.6) | 87(43.3) |
| Secondary | 27(39.7) | 21(30.9) | 25(38.5) | 73(36.3) | |
| Tertiary | 11(16.20 | 6(8.8) | 24(36.9) | 41(21.4) | |
| Residence | Urban | 52(76.50 | 47(69.1) | 58(89.2) | 157(78.1) |
| Rural | 16(23.5) | 21(30.9) | 7(10.8) | 44(21.9) | |
| Alcohol | Yes | 42(61.8) | 35(51.5) | 50(76.9) | 127(63.2) |
| No | 26(38.2) | 33(48.5) | 15(23.1) | 74(36.8) | |
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | ||
| Age | 36.6 ± 9.2 | 42.4 ± 8.0 | 32.9 ± 9.0 | 37.4 ± 9.6 | |
| Body Mass Index | 24.2 ± 3.5 | 25.0 ± 3.9 | 24.7 ± 3.9 | 24.65 ± 3.7 | |
| Systolic Blood Pressure (SBP) | 108.3 ± 14.2 | 116.3 ± 14.2 | 113.6 ± 13.8 | 112.7 ± 14.4 | |
| Diastolic blood pressure (DBP) | 71.0 ± 10.9 | 78.5 ± 9.7 | 73.5 ± 9.4 | 74.4 ± 10.5 | |
| Fasting Blood Sugar (FBS) | 105.0 ± 4.2 | 106.3 ± 4.0 | 104.9 ± 4.7 | 105.32 ± 4.4 | |
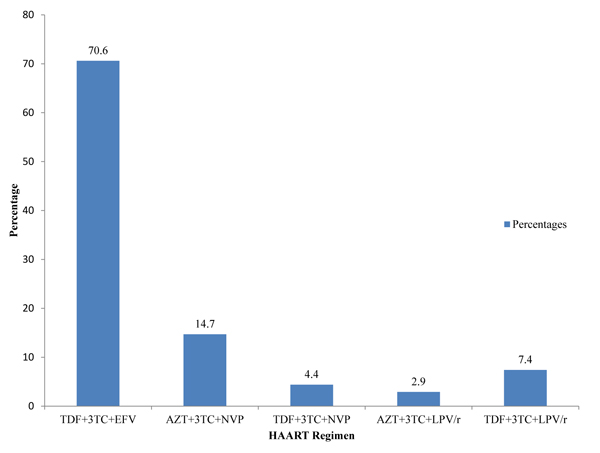
3.2. Prevalence of Proteinuria Among the Study Groups
The study population had a mean 24 hours urine proteins of 206.65±201.17mg/24hours. The cut-off value for a normal 24 hours urine protein was set at <150mg/24hours. Of the total study population, 101(50.2%) participants had normal urine protein concentrations and 100(49.8%) had abnormal urine protein concentrations. The HAART-naïve group had a proteinuria prevalence of 58.8%, HAART experience group had 54.4% and 35.4% for the HIV negative group. Fig. (2) summarizes proteinuria in the study population.
Table 2 below displays the prevalence of renal dysfunction within the three study groups. Of the total study population, 33 (16.4%) had Crcl <60ml/min/1.73m2. The prevalence of renal dysfunction was significantly different among the different study groups (p= 0.022). The HAART experienced group had a higher prevalence of renal dysfunction (26.5%) than the HAART-naive group (11.8%).
3.3. Comparison of Renal Function Among Study Groups
This study demonstrated a significant difference in the mean renal function markers (BUN, BUN-Creatinine ratio, Crcl and Urine protein) among the different study groups (p-values 0.001, 0.003, 0.026 and 0.017 respectively) as shown in Table 3.
Table 4 displays the multiple comparison of the renal function between the study groups using Fisher’s Least Significance difference (LSD). The mean BUN concentration was significantly higher in the HAART group as compared to the HIV negative groups (p= 0.017) and lower in the HAART Naïve group when compared to the HAART groups (p=0.000). The mean BUN-Creatinine was significantly lower in the HAART Naïve than the HAART-experienced group (p= 0.001), mean Crcl significantly decreased in the HAART group when compared to the HIV Negative groups (p= 0.005) and the mean urine protein was significantly higher in the HAART Naïve than in the HIV Negative group (p= 0.007). However, though the HAART-naive group had a higher urine protein and the HAART experience group had decreased Crcl, these differences were not statistically significant (P= 0.150 and 0.053 respectively).
| HAART-naive Frequency(%) |
HAART experienced Frequency(%) |
HIV Negative Frequency(%) |
Chi-square | ||
|---|---|---|---|---|---|
| Creatinine clearance | ≥ 60 | 60(88.2) | 50(73.5) | 58(89.2) | p= 0.022 |
| < 60 | 8(11.8) | 18(26.5) | 7(10.8) |
| Treatment Group | BUN(mg/dl) | BUN/Creatinine ratio | Urine Protein (mg/24hrs) |
Crcl (mL/min/1.73m2) |
|---|---|---|---|---|
| Reference values | 10-20 | 10-20 | <150 | 120-130 |
| HAART-naïve | 14.302±5.21 | 15.787±4.29 | 253.639±282.08 | 92.599±34.60 |
| HAART experienced | 17.453±4.34 | 18.520±4.64 | 204.509±148.47 | 82.090±32.11 |
| HIV negative | 15.487±4.53 | 17.000±5.05 | 159.725±124.50 | 97.443±27.20 |
| Statistical significance | p=0.001 | p=0.003 | p=0.026 | p=0.017 |
| Comparison | BUN (mg/dl) |
BUN-Creatinine | Urine protein (mg/24hours) |
Crcl (mL/min/1.73m2) |
|---|---|---|---|---|
| Reference values | 10-20 | 10-20 | <150 | 120-130 |
| HAART-naïve/ HAART experienced | p< 0.001 | p= 0.001 | p= 0.150 | p= 0.053 |
| HAART-naïve/ HIV negative | p= 0.148 | p= 0.136 | p= 0.007 | p= 0.377 |
| HAART experienced/ HIV negative | p= 0.017 | p= 0.062 | p= 0.195 | p=0.005 |
3.4. Comparison of Renal Functions Among Age Categories
To ascertain whether age could affect the renal function, the renal markers were compared among age categories of the study participants as shown in Table 5. It was demonstrated that the mean BUN and mean Crcl were significantly different among the age groups (p-values were 0.046 and <0.001 respectively).
3.5. Comparison of Renal Functions Among CD4+ Categories
Renal functions in patients with different CD4+ counts were also analyzed as shown in Table 6. Comparing the means of the renal markers between the different CD4 count categories, only the mean BUN level was statistically different between the categories (p= 0.048).
Though there were differences in the mean BUN, BUN-Creatinine ratio, Crcl and Urine proteins between the participants with different durations on HAART, these differences were not statistically significant.
3.6. Relationship Between Renal Function and Adherence/Type of Regimen
This study demonstrated that the renal function markers were not associated with the patients’ adherence to treatment. Pearson’s correlation showed a non-significant correlation between duration on HAART and renal function. CD4 count showed a significant negative correlation with urine protein and positive correlation with BUN (r= 0.200, p= 0.020) and BUN-Creatinine ratio (r= 0.246, p=0.004).
Table 5.
| Age groups | BUN(mg/dl) Mean ± SD |
BUN-Creatinine ratio Mean ± SD |
Creatinine clearance (ml/min/1.73m2) Mean ± SD |
Urine proteins (mg/24l) Mean ± SD |
CD4+ count (Cells/µl) Mean ± SD |
|---|---|---|---|---|---|
| 21 -30 (n= 59) |
14.41 ± 4.33 | 16.85 ± 4.92 | 104.18 ± 34.38 | 193.48 ± 150.25 | 372.04 ± 253.75 |
| 31 -40 (n= 60) |
15.72 ± 5.11 | 16.75 ± 4.82 | 88.08 ± 30.02 | 199.44 ± 279.00 | 413.60 ± 237.76 |
| 41 -50 (n=64) |
16.62 ± 4.87 | 17.62 ± 4.84 | 79.59 ± 25.87 | 198.27 ± 142.95 | 423.51 ± 270.30 |
| >50 (n= 18) |
17.11 ± 5.00 | 17.23 ± 4.14 | 93.70 ± 35.80 | 303.53 ± 205.44 | 546.67 ± 266.58 |
| Statistical significance |
p= 0.046 | p=0.747 | p< 0.001 | p= 0.868 | p= 0.239 |
| CD4 (cells/µL) |
BUN (mg/dl) Mean ± SD |
BUN-Creatinine Mean ± SD |
Crcl (mL/min/1.73m2) Mean ± SD |
Urine protein (mg/24hours) Mean ± SD |
|
|---|---|---|---|---|---|
| <200 (n= 29) 200-500 (n= 58) >500(n= 49) |
14.61±4.41 | 15.90±4.34 | 82.60±29.73 | 286.57±168.01 | |
| 15.36±5.45 | 16.81±4.31 | 86.66±35.35 | 238.09±295.70 | ||
| 17.23±4.60 | 18.29±5.05 | 96.70±35.71 | 184.36±138.22 | ||
| Statistical significance | p-value | p= 0.049 | p= 0.070 | p= 0.199 | p= 0.143 |
4. DISCUSSION
4.1. Prevalence of Renal Dysfunction
This study consisting of HIV outpatients and healthy HIV negative participants attending the Day clinic of the Bamenda Regional Hospital, demonstrated a prevalence of renal dysfunction among HAART-naive patients of 11.8% and 26.5% among HAART experienced patients. The demonstrated prevalence of renal dysfunction in HAART-naïve patients (11.8%) was similar to 14.4% reported by Odongo et al. of Uganda [20]. However, Kahsu et al. of Ethiopia [24] reported a higher prevalence among HAART-naive patients of 30.1%. This difference could be because, instead of the conventional 24 hours urine creatinine clearance as used in this study, Kahsu et al. used the Cockcroft-Gault equation to estimate the creatinine clearance. Hypertensive and diabetic patients included in the Kahsu et al. study could be a reason for this disparity. The prevalence of renal dysfunction among HIV patients on HAART of 26.5% in this study was similar to the 29% reported in a Tanzanian cohort by Mpondo et al. [17]. Nevertheless, this prevalence was higher than the 14.3% reported by Nsagha et al. [14]. This discrepancy could be due to the difference in the method used to estimate the GFR. Nsagha et al. used the modification of diet in renal disease (MDRD) equation to estimate the GFR. The high prevalence of renal dysfunction recorded in this study may also be due to the fact that most of the patients were on Tenofovir-based regimens. Also, the participants on HAART in this study had a high mean blood pressure, age and FBS which could have also contributed to the higher renal dysfunction reported.
4.2. Effect of HAART on Renal Function
In this study, the mean Crcl significantly decreased in HIV patients on HAART when compared to the HIV negative controls, indicating that HAART could have a devastating effect on the kidney. However when renal function was compared between the HAART Naïve and HAART experienced group, there was no significant difference in Urine protein and Crcl. Thus the decreased renal function might only be the effect of HIV. Our result was in line with the observation of Obirikorang et al. who reported renal function to not be associated with the use of HAART [23]. However, a different observation was made from that of Nsagha et al. [14] who reported HAART to be nephrotoxic. This could be due to the fact that Nsagha et al. did not consider HIV infection as a determinant of renal function thereby attributing the cause of renal dysfunction entirely to the effect of HAART. Patients on HAART had a higher mean BUN and BUN-Creatinine ratio when compared to the HAART-naive patients. This result was different from the report of Kahsu et al. [24] who demonstrated that HAART-naive participants have a higher mean BUN. Our study demonstrated high proteinuria among HAART-naïve participants of 58% when compared to HIV negative participants, which could mean that HIV increases the risk of developing proteinuria. It was demonstrated that though the prevalence of proteinuria was higher in the HAART-naïve participants than the HAART experienced participants, this difference in mean urine protein was not significant between these two categories. This could further signify that HAART does not reverse the damages caused by HIV. Our prevalence of 58% was not in line with Kaze et al. [25] of Cameroon who reported a prevalence of 36%. This disparity could have been because, instead of the gold standard 24 hours urine protein as used in this study, the dipstick urinalysis was used in the study by Kaze et al. which is less sensitive.
4.3. Effect of CD4 Count and Age on renal Function
This study demonstrated older age to be associated with decreased Crcl. This was in agreement with the report of Mpondo et al. [17] where age also reported to be associated with a decrease in renal function. This study also demonstrated no association between decreased renal function and the type of HAART regimen, the duration on HAART as well as the CD4 counts. This was in agreement with the report of Kamga et al. [26] who reported that the effect of HAART on the renal function was irrespective of the HAART regimen used, the duration of treatment or the CD4 counts. Our result was not in agreement with that reported by Nsagha et al. [14] who reported the HAART regimen tenofovir, lamivudine and efavirenz to be the most nephrotoxic. This study also reported no association between decreased renal function and patient’s adherence to treatment.
This study can be considered limited as microalbumin was not measured, thus a total lower urine protein was not included. However, a quantitative measurement of urine proteins gives a good picture of the renal function. Another limitation in this study was that the evaluation of proteinuria after 3 months was not repeated to differentiate acute from chronic kidney disease; nonetheless, the BUN-Creatinine ratio was calculated which could serve this purpose.
CONCLUSION
This study demonstrated that HAART is associated with decreased creatinine clearance, increased blood urea nitrogen and blood urea nitrogen to creatinine ratio. However, there was no significant difference in renal function between HIV infected patients not yet on HAART and those who were already on HAART. This is indicative that this decrease in renal function could have been as a result of the devastating effect of HIV. This study also demonstrated no association between the kidney function tests and the duration, type of HAART regimen and adherence to treatment. This could emphasize that the decreased renal function among the patients on HAART may only be the effect of HIV.
LIST OF ABBREVIATIONS
| BMI | = Body Mass Index |
| BUN | = Blood Urea Nitrogen |
| CD4 | = Cluster of Differentiation 4 |
| Crcl | = Creatinine Clearance |
| eGFR | = Estimated Glomerular Filtration Rate |
| HAART | = Highly Active Antiretroviral Therapy |
| KDIGO | = Kidney Disease International Global Outcome |
| MDRD | = Modification of Diet in Renal Disease |
| PLWHA | = Peopleliving with HIV/AIDS |
| WHO | = World Health Organization |
AUTHORS’ CONTRIBUTIONS
ACAN conceived, designed, conducted the study, collected and analyzed the data, conducted the literature search and review, and wrote the manuscript. BRN assisted in study design and review of the manuscript. EAT and FSW assisted in Data analysis and critically reviewed the manuscript. AEA and NJCA coordinated the entire study and reviewed the manuscript. All authors read and approved the final paper.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Ethical clearance was obtained from the Bamenda Regional Hospital Institutional Review Board (BRHIRB 2016/023). Authorization was obtained from the North West Regional Delegation of Public Health and from the administration of the Bamenda Regional Hospital ,Cameroon.
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All human research procedures were followed in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
A written consent form was provided to the participants wherein the procedure of the study was explained, its risks and benefits to the participant, confidentiality and the participant’s right of withdrawal from the study at any time. Only participants who gave their consent by signing the consent form were recruited into the study.
AVAILABILITY OF DATA AND MATERIALS
All data generated or analysed during this study are included in this published article.
FUNDING
We acknowledge the financial assistance from the Adolphe Monkiedje Fellowship eV board, (2015(16)) to carry out this study.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
This is part of a M.Sc thesis by Achu Che Awah under the supervision of Prof. Assob and co-supervision of Prof. Asongalem in the Department of Medical laboratory Sciences of the University of Buea. The authors are grateful for the participation of the entire staff of the Day clinic and laboratory of the Bamenda Regional Hospital to the realization of this study. We as well appreciate all our participants for their patience and dedication. We acknowledge the financial assistance from the Adolphe Monkiedje Fellowship board to carry out this study.


