All published articles of this journal are available on ScienceDirect.
Meta-Analysis of The Prevalence of Genital Infections Among Hiv Carriers and Uninfected Women
Abstract
Background & Aim:
The risk factors in acquiring genital co-infections associated with HIV infection still present many questions. We conducted a systematic review and meta-analysis to compare the prevalence of genital infection among HIV-infected and uninfected women.
Methods:
We searched PubMed, Web of Science, Scopus and Scielo for the relevant studies up until October 2017. Data were collected from the included studies and methodologically assessed. Odds ratios (OR) and 95% confidence intervals (CI) were pooled using fixed or random-effects models.
Results:
Thirty-six articles involving 23,863 women with retroviruses were included. HIV-infected women were significantly more diagnosed with the following genital infections: Herpes simplex virus type 2 (HSV-2) (OR 3.70; 95% CI: 2.42–5.65), Neisseria gonorrhoeae (GC) (OR 4.18; 95% CI: 2.15-8.13), Chlamydia trachomatis (CT) (OR 2.25; 95% CI: 1.20-4.23) and Human papillomavirus (HPV) (OR 3.99, 95% CI: 3.35-4.75). There was no significant difference in the prevalence of bacterial vaginosis (OR 1.09; 95% CI: 0.91-1.30), Candida sp. (OR 1.51; 95% CI: 0.71-3.25), Treponema pallidum (OR 1.56; 95% CI: 1.00-2.45) and Trichomonas vaginalis (OR 1.00; 95% CI: 0.47-2.15).
Conclusion:
The prevalence of HPV, HSV-2, GC and CT genital infection was significantly higher among HIV-positive women.
1. INTRODUCTION
The microflora of healthy vaginal mucosa is typically dominated by the Lactobacillus species, which serve as an important natural barrier against infection [1]. Clinical studies have shown an association between the presence of lactobacillus and a decrease in the prevalence of gonorrhea, bacterial vaginosis and Human Immunodeficiency Virus (HIV) infection [2, 3].
Recently, many epidemiological studies have highlighted the increase in the prevalence of genital infection in certain sub-populations of women. There have been fewer publications addressing the changing epidemiology of Sexually Transmitted Diseases (STD) among women, and these publications have demonstrated that the risk of STD has significantly increased among HIV-positive women [4].
Sexually transmitted infections are a major public health concern in many developing countries. Women are more likely to bear the burden of these infections but less likely to seek medical treatment. The World Health Organization (WHO) estimates that globally, there are about 340 million new cases of curable STDs (Chlamydia, gonorrhea, syphilis and trichomoniasis) each year [5].
There are gaps in the knowledge of how certain STD or microbiota imbalances can facilitate the HIV infection. It is well accepted that Trichomonas vaginalis infection and bacterial vaginosis can lead to changes in the innate and adaptive immune response in women’s lower genital tract through the presence of microbial products, induction of proinflammatory cytokines, cell recruitment and loss of epithelium integrity. These alterations are associated with an increase in HIV infection rates related to these pathogens [6, 7].
On the other hand, the knowledge regarding risk factors for acquiring genital co-infections associated with HIV infection is still incomplete and many associations are still considered controversial, as is the case for the association of vulvovaginal candidiasis with HIV. Women with recurrent candidiasis are often recommended to test for HIV, however, some authors maintain that there is no direct association between candidiasis and HIV infection and that the clinical manifestation is no different from that observed in HIV uninfected women [8, 9].
The aim of this meta-analysis is to compare the prevalence of some genital infections among HIV carriers and uninfected women.
2. MATERIALS AND METHODS
2.1. Study Design
This meta-analysis was designed and reported according to the modified Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [10]. Since this study was based on previously published studies, no ethical approval and patient consent was required.
2.2. Search Strategy
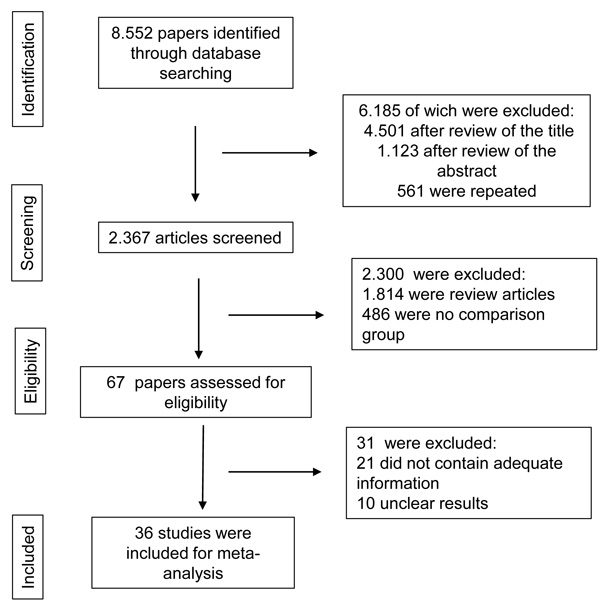
The literature searches were conducted in the following electronic databases: PubMed, Web of Science, Scopus and Scielo from January 1991 to October 2017. The following MeSH terms and keywords were used: [HIV] and [HIV infection] and [Sexually Transmitted Diseases] and [microbiome] or [vaginal microbiome] or [cervicovaginal microbiota] or [vaginal diseases] or [vaginitis] and [symptoms] and [women]. No language restrictions were applied and four independent researchers (APFC, ACAS, PHAS and MGS) performed this search. The flowchart of this study is shown in Fig. (1).
All potentially relevant publications were then evaluated by three individuals (APFC, MGS and RNOC) and were included in this review if they addressed any of the following criteria: (1) Cross-sectional and cohort studies involving only non-pregnant women and (2) Comparing prevalence of genital infections among women with and without HIV. Publications were excluded if they were case reports, reviews, included men and did not include original data. Reference lists of included studies were manually screened to identify other relevant publications. Disagreements were resolved by means of mutual consensus.
2.3. Data Extraction
Table 1 lists the studies included in this review. Study characteristics (author, publication date, study design, period, types of microorganisms and diagnosis tests used) and study population (study location, number and age range of participants) were extracted from all included studies. We identified peer-reviewed publications that included the following criteria: population: women diagnosed with HIV; intervention: diagnostic tests to assess the presence of genital infection; control: women not infected with HIV; outcome: prevalence of genital infection. We only included Cross-sectional and cohort studies which covered all the points of the PICO strategy. When duplicate publications or secondary publications with overlapping patient populations were detected, the authors were contacted to collect non-overlapping data for inclusion in the meta-analysis. Three reviewers (JCOC, AKG and GMC) extracted the data from all articles and any disagreement was resolved by consensus.
| Author, Year | Country | Design of Study | Types of Microorganisms | Methodology | Follow-up | Age Range (y) | Sample Size |
|---|---|---|---|---|---|---|---|
| Reference | |||||||
| Vermund, et al. 1991 [19] | USA | Cross-sectional | HPV | PCR | - | 23-58 | 97 |
| Miott, et al. 1996 [21] | Malawi | Cross-sectional | HPV | PCR | 1 year | - | 284 |
| Chiasson, et al. 1997 [47] | USA | Cross-sectional | HPV | PCR | - | >18 | 771 |
| Uberti-foppa, et al. 1998 [23] | Italy | Cross-sectional | HPV | PCR | 1 year and 10 months | 21-64 | 268 |
| Cu-uvin, et al. 1999 [2] | USA | Cohort | BV | Clinical examination | 1 year and 9 months | 16-55 | 1.31 |
| HPV | PCR | ||||||
| Candida sp. | Culture | ||||||
| N. gonorrhoeae | Serology | ||||||
| Treponema pallidum | Culture | ||||||
| T. vaginalis | Serology | ||||||
| Chlamydia trachomatis | |||||||
| Warren, et al. 2001 [39] | USA | Cohort | Gardnerella sp. | Gram | 1 year and 9 months | 16-55 | 1.31 |
| BV | |||||||
| Conley, et al. 2002 [22] | USA | Cohort | HPV | PCR | 6 years and 11 months | 16-35 | 925 |
| Branca, et al. 2003 [26] | Italy | Cohort | HPV | PCR | 2 years | 17-45 | 142 |
| Viscidi, et al. 2003 [27] | USA | Cohort | HPV | PCR | 1 year and 9 months | 16-55 | 2.559 |
| Viscidi, et al. 2003b [28] | USA | cohort | HPV | PCR | 1 year and 9 months | 16-55 | 2.524 |
| Ohmit, et al. 2003 [44] | USA | Cohort | Candida sp. | Culture | 1 year and 9 months | 16-55 | 1.31 |
| Harris, et al. 2005 [29] | USA | Cohort | HPV | PCR | 1 year | 34-36 | 1.198 |
| Watts, et al. 2005 [24] | USA | Cohort | BV | Nugent criteria | 1 year and 1 month | - | 2.229 |
| HPV | PCR | ||||||
| T. vaginalis | Fresh examination | ||||||
| Watts, et al. 2006 [25]. | USA and Hawaii | Cohort | BV | Gram | 1 year and 1 month | - | 2.652 |
| HPV | PCR | ||||||
| T. vaginalis | Fresh examination | ||||||
| Didelot-rousseau, et al. 2006 [31]. | Bobo-Dioulasso | Cross-sectional | HPV | PCR | - | 16-54 | 379 |
| Ng´andwe, et al. 2007 [32] | Zambia | Cohort | HPV | PCR | 1 year and 1 month | 15-38 | 70 |
| sha 2007 [16] | USA | Cohort | BV | Nugent criteria | 1 years and 1 month | - | 2.628 |
| Candida sp. | Smear with potassium hydroxide (KOH) | ||||||
| T. vaginalis | Fresh examination | ||||||
| Singh, et al. 2009 [33] | Rwanda | Cohort | HPV | PCR | 6 months | >25 | 936 |
| Desruisseau, et al. 2010 [35] | Camaroon | Cross-sectional | HPV | PCR | 10 years and 11 months | >18 | 65 |
| Djigma, et al. 2011 [34] | Burkina Faso | Cohort | Mycoplasma sp. | PCR | 6 months | 27-45 | 451 |
| Candida sp. | Culture | ||||||
| N. gonorrhoeae | |||||||
| Nowark, et al. 2011 [45]. | Zimbabue | Cohort | HSV-2 | ELISA | 4 years and 2 months | 28-35 | 641 |
| BV | AMSEL CRITERIA | ||||||
| HPV | PCR | ||||||
| N. gonorrhoeae | |||||||
| Chlamydia trachomatis | |||||||
| Gallo, et al. 2011 [40] | USA | Cohort | BV | Nugent criteria | 2 years | 16-55 | 1364 |
| Oliveira, et al. 2011 [17] | Brazil | Cross- sectional | Candida sp. | Culture | 1 year | >18 | 140 |
| Chiduo, et al. 2012 [30] | Tanzania | Cohort | Candida sp. | Gram | 2 years | >18 | 205 |
| Treponema pallidum | Serology | ||||||
| T. vaginalis | Fresh examination PCR | ||||||
| Chlamydia trachomatis | |||||||
| Blitz, et al. 2013 [36] | Canada | Cohort | HPV | PCR | 9 years | 15-44 | 923 |
| Hu, et al. 2013 [18] | USA | Cohort | Candida sp. | Culture | 4 years and 5 months | >18 | 24 |
| Apalata, et al. 2014 [20] | South Africa | Cohort | HSV-2 | PCR | 7 months | >18 | 198 |
| Mycoplasma sp. | Culture | ||||||
| Candida sp. | PCR | ||||||
| N. gonorrhoeae | |||||||
| T. vaginalis | |||||||
| Chlamydia trachomatis | |||||||
| Benning, et al. 2014 [41] | USA and Rwandan | Cohort | N. gonorrhoeae | Sequencing | 1 years and 10 months | 33-51 | 86 |
| Mackelprang, et al. 2015 [43] | USA | Cross-sectional | BV | Nugent criteria | - | - | 372 |
| Masson, et al. 2015 [42] | South Africa | Cross-sectional | HSV-2 | ELISA | - | 18-59 | 265 |
| BV | Nugent criteria | ||||||
| Mycoplasma sp. | PCR | ||||||
| N. gonorrhoeae | Quick test | ||||||
| Treponema pallidum | PCR | ||||||
| T. vaginalis | |||||||
| Chlamydia trachomatis | |||||||
| Alczuk, et al. 2015 [15] | Unganda | Cohort | Candida sp. | Culture | 4 years and 2 months | 18-35 | 641 |
| Massad, et al. 2016 [37] | USA | Cohort | HPV | PCR | 8 years | >18 | 3438 |
| Reimers, et al. 2016 [38] | USA | Cohort | HPV | PCR | 8 years | >18 | 64 |
| Kriek, et al. 2016 [46] | South Africa | Cohort | HPV | PCR | 6 months | 28-42 | 165 |
| Mycoplasma sp. | |||||||
| N. gonorrhoeae | |||||||
| T. vaginalis | |||||||
| Chlamydia trachomatis | |||||||
| Alcaide, et al. 2016 [14] | USA | Cross-sectional | BV | Nugent criteria | 8 months | 18-45 | 34 |
2.4. Risk of Bias Assessments
The Cochrane Collaboration tool for assessing the risk of bias was applied to evaluate the following criteria: The adequate sequence generation, allocation concealment, blinding, incomplete outcome data, selective reporting and other risks of bias [11]. Two authors (ACAS and PHAS) assessed each original study and the quality of the accurate information of the studies was demonstrated in Table 2. Each of the previously cited criteria received one of the following classifications: “low risk of bias”, “high risk of bias”, or “unclear risk of bias”. Disagreements were resolved by consulting a third author (RNOC).
2.5. Data Analysis
Dichotomous data from each of the eligible studies were combined for meta-analysis using the Mantel/Haenszel model. Results were expressed as Odds Ratios (OR) with 95% Confidence Intervals (CI). Fixed-effects or random-effects models were chosen depending on whether there was an absence or presence of heterogeneity between studies. Statistical heterogeneity was assessed by the I2 statistic (<25%, no heterogeneity; 25%–50%, moderate heterogeneity; and >50%, strong heterogeneity). When a significant heterogeneity existed across the included studies (I2 > 50%), a random-effects model was used for the analysis; otherwise, the fixed-effects model was used [12, 13]. Meta-regression was conducted to investigate the potential heterogeneity. In addition, we used the Egger funnel plot to assess possible publication bias (12). All tests were performed using Review Manager (RevMan version 5.3.0) software and two-sided p value < 0.05 was considered statistically significant.
| Study/Year Reference |
Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Other bias |
| Vermund 1991 [19] |  |
 |
 |
 |
 |
 |
 |
| Miott 1996 [21] |  |
 |
 |
 |
 |
 |
 |
| Chiasson 1997 [47] |  |
 |
 |
 |
 |
 |
 |
| Uberti-Foppa 1998 [23] |  |
 |
 |
 |
 |
 |
 |
| Cu-Uvin 1999 [2] |  |
 |
 |
 |
 |
 |
 |
| Warren 2001 [39] |  |
 |
 |
 |
 |
 |
 |
| Conley 2002 [22] |  |
 |
 |
 |
 |
 |
 |
| Branca 2003 [26] |  |
 |
 |
 |
 |
 |
 |
| Viscidi 2003 [27] |  |
 |
 |
 |
 |
 |
 |
| Viscidi 2003b [28] |  |
 |
 |
 |
 |
 |
 |
| Ohmit 2003 [44] |  |
 |
 |
 |
 |
 |
 |
| Harris 2005 [29] |  |
 |
 |
 |
 |
 |
 |
| Watts 2005 [24] |  |
 |
 |
 |
 |
 |
 |
| Watts 2006 [25] |  |
 |
 |
 |
 |
 |
 |
| Didelot-Rousseau 2006 [31] |  |
 |
 |
 |
 |
 |
 |
| Ng´andwe 2007 [32] |  |
 |
 |
 |
 |
 |
 |
| Sha 2007 [16] |  |
 |
 |
 |
 |
 |
 |
| Singh 2009 [33] |  |
 |
 |
 |
 |
 |
 |
| Desruisseau 2010 [35] |  |
 |
 |
 |
 |
 |
 |
| Djigma 2011 [34] |  |
 |
 |
 |
 |
 |
 |
| Nowark 2011 [45] |  |
 |
 |
 |
 |
 |
 |
| Gallo 2011 [40] |  |
 |
 |
 |
 |
 |
 |
| Oliveira 2011 [17] |  |
 |
 |
 |
 |
 |
|
| Chiduo 2012 [30] |  |
 |
 |
 |
 |
 |
 |
| Blitz 2013 [36] |  |
 |
 |
 |
 |
 |
 |
| Hu 2013 [18] |  |
 |
 |
 |
 |
 |
 |
| Apalata 2014 [20] |  |
 |
 |
 |
 |
 |
 |
| Benning 2014 [41] |  |
 |
 |
 |
 |
 |
 |
| Mackelprang 2015 [43] |  |
 |
 |
 |
 |
 |
|
| Masson 2015 [42] |  |
 |
 |
 |
 |
 |
 |
| Alczuk 2015 [15] |  |
 |
 |
 |
 |
 |
 |
| Massad 2016 [37] |  |
 |
 |
 |
 |
 |
 |
| Reimers 2016 [38] |  |
 |
 |
 |
 |
 |
 |
| Kriek 2016 [46] |  |
 |
 |
 |
 |
 |
 |
| Alcaide 2016 [14] |  |
 |
 |
 |
 |
 |
 |
 :High risk of bias:
:High risk of bias:  Unclear risk of bias:
Unclear risk of bias:  Low risk of bias
Low risk of bias
3. RESULTS
A total of 36 relevant published articles were included for meta-analysis, involving 23,863 HIV-infected women [2, 14-47] Table 1. Among those infected by HIV there was a significantly higher prevalence of genital infection by certain microorganisms compared to uninfected women.
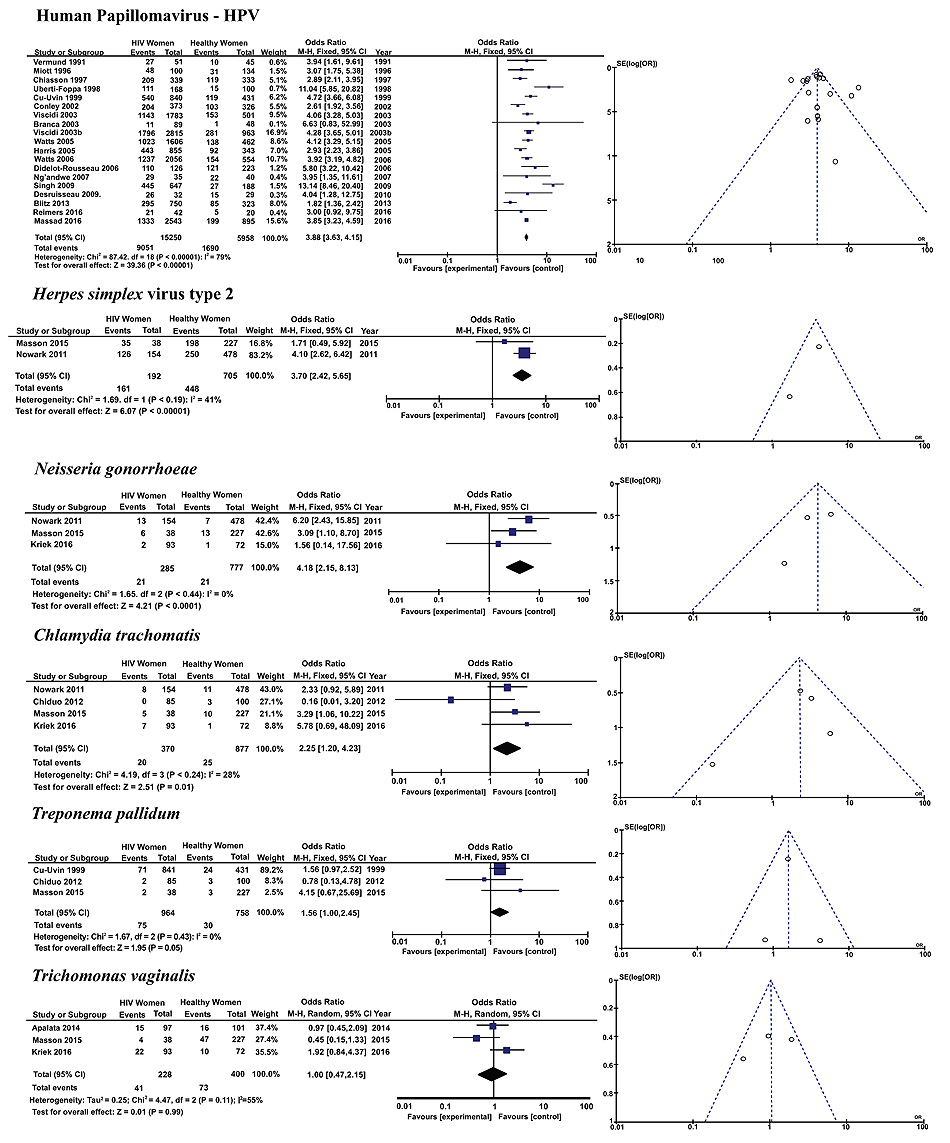
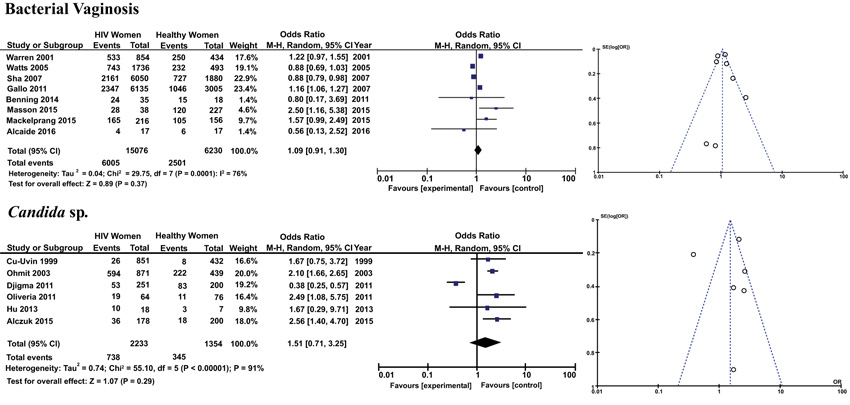
3.1. Subgroup Analysis
Subgroup analysis was carried out for microorganisms that cause STD (Fig. 2) and for those found in the microbiota of the female genital tract (Fig. 3), which cause genital infection when there is an imbalance of the vaginal microflora.
3.1.1. Human Papillomavirus (HPV)
The prevalence of HPV (OR 3.99, 95% CI: 3.35-4.75) in the reproductive tract was significantly higher in HIV-infected women than in similarly aged control women.
3.1.2. Herpes Simplex Virus Type 2 (HSV-2)
Similarly, HSV-2 (OR 3.70; 95% CI 2.42-5.65) genital infection was more prevalent among retrovirus carriers.
3.1.3. Neisseria Gonorrhoeae (GC)
The diagnosis of GC (OR 4.18; 95% CI 2.15-8.13) was also significantly higher among HIV-infected women.
3.1.4. Chlamydia Trachomatis (CT)
Vaginal and cervical CT infections (OR 2.25; 95% CI 1.20-4.23) were significantly more prevalent among those infected with HIV.
3.1.5. Treponema Pallidum (TP)
Among the Sexually Transmitted Infections (STI) investigated in the reproductive tract of women of both groups, there was not a higher prevalence of TP infection (OR 1.56; 95% CI 1.00-2.45), the causative agent of syphilis.
3.1.6. Trichomonas Vaginalis (TV)
TV (OR 1.00; 95% CI 0.47-2.15) was the other microorganism transmitted through sexual contact whose prevalence was no different between the groups.
3.1.7. Bacterial Vaginosis (BV)
The prevalence of microorganisms causing BV (Gardnerella vaginalis, Mycoplasma sp., etc.), not considered a STI, was not significantly different between the groups (OR 1.09; 95% CI 0.91-1.30).
3.1.8. Candida sp.
Finally, there was no significant difference in fungal infection by different Candida species in the genital tract of women in both groups (OR 1.51; 95% CI 0.71-3.25).
3.1.9. Publication Bias
Potential publication bias was not presented according to the funnel plots, which are shown in (Figs. 2 and 3).
3.1.10. Meta-Regression
Using meta-regression there was little evidence of heterogeneity between any of these subgroups (p >0.05 by meta-regression).
4. DISCUSSION
This meta-analysis found that HIV carriers are more likely to be diagnosed with certain genital infections than women not infected with the virus. Subgroup analysis showed that the prevalence of most STIs is significantly higher among women with HIV, while infections caused by microorganisms of the vaginal microbiome are no longer prevalent in this group.
HIV can affect the immunological host response, resulting in an increased susceptibility to STIs. In women, this association was observed mainly in the presence of microorganisms causing intraepithelial lesions as well as cervicitis, which disturbs the epithelial barrier, causing local inflammation and increases the viral load of the genital tract [3, 48].
In this meta-analysis, the prevalence of STI was reported in all the analyzed studies [2, 14-47]. The most reported infections were HPV, Candida sp., BV, T. pallidum, T. vaginalis, N. gonorrhoeae, C. trachomatis and HSV-2. The susceptibility to STI may increase because of the negative effects of HIV infection on the immunity system, which is often unable to mount a protective response against sexually transmitted pathogens [49]. In line with this, HIV positive women were significantly more infected by HPV, HSV-2, GC and CT, compared to women not infected by the virus.
HPV genital infection can lead to cervical cancer. Co-infection with HIV increases the burden of HPV infection [50]. HIV-positive women are more vulnerable to HPV infection and have a lower propensity to eliminate this virus, increasing the risk of developing lesions and cancer [47]. These women, especially those with lower CD4 cell counts, have a higher prevalence of anal and cervical HPV infection and HPV cancers [51]. HPV detection by PCR has significantly increased in HIV positive women, as in a previous meta-analysis that indicated a higher prevalence of high-risk type HPV-16 in HIV infected women, and a higher incidence of multiple HPV infection in this group, with a consequent higher rate of high grade lesions [50, 52, 53].
Bacterial vaginosis and Trichomonas vaginalis infection are very common and are associated with an increased risk of sexual transmission of HIV. Previous studies [54-59] indicated that BV was associated with an increased risk of HIV transmission, probably as a result from an imbalance in the immune response, loss of epithelial integrity and an increase in HIV shedding, especially when associated with TV infection. It was previously reported that HIV infected women with controlled viral load might be less likely to have BV, possibly because those women are more likely to abstain from sex or to use condoms [60]. Bacterial vaginosis data was determined by the Nugent criteria in all selected studies and there was no significant difference in the prevalence among the groups. Moreover, despite the role played by T. vaginalis in HIV transmission, laboratory detection of the parasite was not more frequent in HIV positive women, suggesting that the immunological response against this infection is not impaired by the HIV presence.
The frequency of vulvovaginal candidiasis (VVC) caused by Candida species, such as Candida albicans, Candida tropicalis, Candida glabrata, and Candida krusei is increasing, especially in HIV-infected women [61]. However, the prevalence of VVC was not higher among HIV-infected women in this meta-analysis, in agreement with previous reports [20], which indicate that the HIV load is not associated with an increased risk of symptomatic VVC.
Sexually transmitted pathogens, such as Neisseria gonorrhoeae (GC) and Chlamydia trachomatis (CT) are associated with increased HIV particles in genital secretions. During chronic HIV infection, GC infection was associated with a transient increase in plasma viremia and cytokines, such as IL-4 and IL-10, and a decrease in CD4+ T cell counts [62]. A previous study from Brazil reported higher rates of GC among HIV-infected women [63]. Moreover, it was previously reported that there was a higher frequency in antibiotic-resistant strains in patients co-infected with GC and HIV, possibly a consequence of repeated treatments [64]. Likewise, studies indicate that genital CT infection is more prevalent in women with the virus, corroborating the findings of this study [65, 66].
Syphilis is an emerging STD that has been growing in recent years especially among people with HIV. Syphilis and HIV appear to be the most commonly reported STDs [67]. Some studies have shown that the prevalence of syphilis among women is increasing worldwide [68]. In this meta-analysis, the prevalence of T. pallidum infection was no different between the groups, in agreement with the findings of previous studies [69, 70].
The decrease in the host's innate or adaptive immune response results in infectious clinical conditions, which may be persistent or disseminated. Persistent HSV-2 is a common presentation of HIV type 1 (HIV-1) advanced infection; low CD4 counts and high viral load facilitate the emergence of HSV-2 infections [71-74]. This fact justifies the higher prevalence of the herpes virus in the genital tract of women with HIV, as verified in this study.
Some limitations in our study should be addressed. First, as with any systematic review and meta-analysis, there was the unavoidable heterogeneity among different studies, which could distort the combined estimates. Secondly, another potential limitation of the study was the various methods used to diagnose STI among the different studies. Thirdly, all the included studies were observational studies, which can introduce recall bias or selection bias. Finally, our study did not address the issues related to the participants’ disease history at baseline, since the studies included had no information regarding sexual behavior, CD4 and CD8 levels, and the severity of HIV infection in HIV-infected women, which has been shown to influence the risk of genital infection. Furthermore, one possibility is that women living with HIV who have been evaluated for the prevalence of STD in studies conducted after the introduction of Antiretroviral Therapy (ART) have less infection than those assessed prior to therapy. However, the purpose of the meta-analysis was to compare the prevalence of infections among HIV-infected and non-HIV-infected women, and because most of the included studies are cross-sectional, we cannot establish the relationship between ART use and the decrease or increase in infection diagnosis.
CONCLUSION
This meta-analysis demonstrated that individuals with HIV had a significantly increased prevalence of syphilis, HPV, HSV-2, N. gonorrhoeae and C. trachomatis. However, owing to the inherent limitations of the included studies, further high-quality, prospective, and multicentered studies are needed to confirm our result.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


