All published articles of this journal are available on ScienceDirect.
Cervical HPV Infection in Female Sex Workers: A Global Perspective
Abstract
Introduction:
Approximately 291 million women worldwide are HPV DNA carriers. Studies have indicated that having multiple sexual partners may lead to higher HPV transmission. Thus female sex workers (FSWs) may be at greater risk of infection compared to the general population. Herein we review publications with data on FSW cervical HPV test results. We also examine variations of HPV prevalence and risk behaviors by region. Knowledge of prevalent HPV types in FSWs may lead to improved prevention measures and assist in understanding vaccination in high-risk groups.
Methods:
We conducted a review of the literature by searching PUBMED using the terms “prostitution” or “female sex workers”, “human papillomavirus” or “HPV”, and “prevalence” or “PCR” to find articles. We excluded studies without HPV testing or HPV type specific results, or unconventional HPV testing.
Results:
A total of 35 peer-reviewed publications were included in our review. High risk HPV types 16 and 18 ranged from 1.1-38.9‰ in prevalence. In addition to high-risk HPV types, newer studies reported non-carcinogenic HPV types also of high prevalence. The most prevalent HPV types reported among FSWs included HPV 6 (11.5%), 16 (38.9%), 18 (23.1%), 31 (28.4%), 52 (32.7%), and 58 (26.0%).
Conclusions:
Female sex workers have an overall high prevalence of HPV infection of high-risk types as evident through various testing methods. FSWs are thought to be at increased risk of cervical cancer because of high HPV exposure. This highlights the need for HPV and cervical prevention campaigns tailored to FSWs.
INTRODUCTION
The Human Papilloma Virus (HPV) encompasses a family of viruses that can infect the genital tract and cervix of women. It is a known causal factor in the development of cervical cancer and genital warts [1]. Approximately 291 million women worldwide are HPV DNA carriers [2]. The virus is associated with anal, vaginal, penile and oral cancers [3]. It is most commonly associated with cervical cancer as the virus causes nearly 500,000 incident cases of cervical cancer and 274,000 cervical cancer deaths annually [4]. Cervical cancer is the second most frequently diagnosed type of cancer in women worldwide. It is much more common in developing countries, where 83% of cases occur [5]. Studies have indicated that a high number of lifetime partners may lead to a higher transmission of HPV leading to higher cervical cancer rates [6-8]. In female sex workers (FSWs), the risk of HPV infection and cervical cancer is especially high. A previous studies have reported that FSW had more than twice the probability of having HPV infection than women from the general population and have a higher prevalence of abnormal pap smears [9, 10]. In addition, HPV can be transmitted from FSWs to the general population through clients thereby increasing the prevalence of the virus.
There are currently two approved HPV vaccines on the market. The vaccines produced by Merck (quadrivalent HPV vaccine) and GlaxoSmithKline (bivalent HPV vaccine) both protect against chronic infection with HPV types 16 and 18, whereas the quadrivalent HPV vaccine also protects against HPV types 6 and 11 among individuals unexposed to the virus. HPV types 6 and 11 cause 90% of genital warts and types 16 and 18 cause 70% percent of cervical cancers worldwide [11]. Approximately 32% of the 291 million HPV infected women from the general population are infected with types 16 and 18 [2]. However there is limited information on the most prevalent HPV types for the FSW population. To our knowledge there have been only two published review papers on sexually transmitted infections (STIs) in FSWs. One review reported results from only 2 studies that assessed HPV in this population [12], whereas the other review focused on FSWs in Asia [13]. In order to assess the burden of HPV infection in the FSW population, we reviewed articles that assessed the prevalence of HPV types in the FSW population worldwide, and the association of HPV with cervical cancer precursors in this population.
With an understanding of the prevalence of HPV in each region, better screening methods can be developed and utilized that provide more accurate results for the HPV types prevalent in said region. Eventually, HPV vaccines can be tailored to prevent infection with region-specific HPV types.
METHODS
We conducted a review of the literature by searching PUBMED using the terms “Prostitution” or “female sex workers”, “human papillomavirus” or “HPV”, and “prevalence” or “PCR” to find articles published from November 9, 1989 to November 9, 2012.
We excluded studies without HPV testing results, type specific prevalence rates or only reported multiple infection prevalence rates. We also excluded studies based on their methodology including studies that tested less than 5 FSWs, tested only HIV positive participants, or used unconventional methods of HPV sample collection (tampon). We only included studies that were written in English or Spanish due to the mother tongue of the authors. Original articles that were unavailable, opinion papers, commentaries, case reports, systematic reviews and subsamples from original papers were not reviewed herein, nor were studies that used any method of sample collection other than cervical or vaginal swabs or scrapes. Additionally, we identified significant references in existing review papers and subsequently excluded the review paper itself.
RESULTS
This PUBMED search resulted in a total of 127 manuscripts (Fig. 1). Overall, fourteen papers were excluded because they were in a language other than English or Spanish, the original paper was not available or were out of the predetermined date range. One hundred and thirteen full text manuscripts were screened. Of these, 78 papers were subsequently excluded because they were a commentary or opinion paper, case report, referenced another paper, review paper and we found the original article [12,14], non-relevant review paper, or was a subsample from another paper in our review. We also excluded papers that had no data on female sex workers, an insufficient number of sex workers, used unconventional HPV testing, no data on HPV or type specific results. A total of 35 eligible manuscripts were included in this review, including four case control studies, one cohort study, and 30 cross sectional studies [9, 15-48]. We included 6 studies from Africa, 6 from the Americas, 2 from Eastern Mediterranean, 10 from Europe, 2 from Southeast Asia and 9 from the Western Pacific Region. For this review, studies that reported the prevalence of type specific single infections among FSWs were used. Numerous studies included both HIV positive and negative FSWs. Data that only presented multiple, coexisting infection data or HIV positive FSWs data was not included. Background information from studies included in the review including sample size and HPV types detected is shown in Table 1.
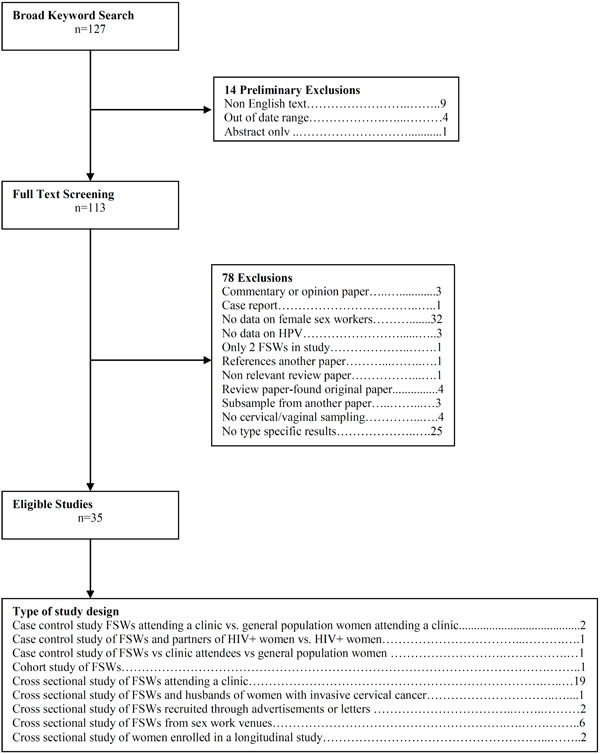
Flow chart.
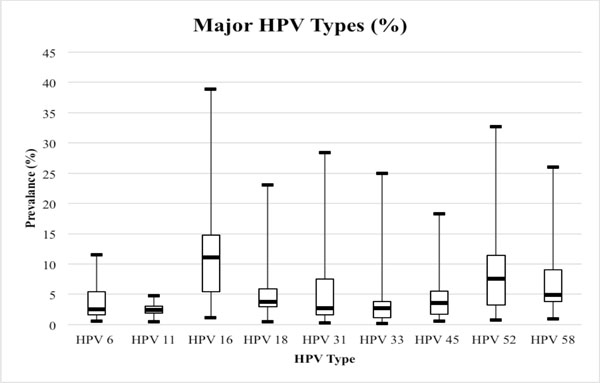
Range of HPV DNA prevalence in 35 studies by 9 major HPV genotypes. Legend: Boxplot markers represent minimum, quartile 1, median, quartile 3 and maximum HPV type prevalence.
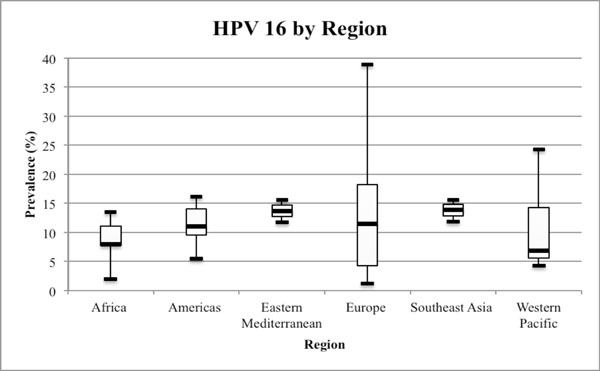
Range of HPV 16 in the WHO defined regions. Legend: Boxplot markers represent minimum, quartile 1, median, quartile 3 and maximum HPV 16 prevalence.
Background Information on 35 Studies of Female Sex Workers that Include HPV Genotype Testing
| WHO Defined Region-Date | Country | City or State | # FSWs | HPV Sampling and Testing Methods | HPV Types Tested | Overall HPV Prevalence |
|---|---|---|---|---|---|---|
| Africa-2007 [31] | Burkina Faso | Bobo-Dioulasso | 350 | Samples obtained with a cervical brush. HPV DNA was detected with PCR and genotyped with INNO LiPA (Line Probe Assay) HPV Genotyping v2 test | 16, 18, 31, 35, 51 and 52 | 8.0% |
| Africa-2010 [29] | Kenya | Mombasa | 503 | Samples obtained with a cervical brush. HPV DNA was detected and genotyped with real time PCR and TaqMan | 6, 16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 67 and 68 | 45.5% |
| Africa-2006 [16] | Burkina Faso | Bobo Dioulasso | 360 | Samples obtained with a cervical brush. HPV DNA was detected with PCR and genotyped with INNO-LiPA HPV genotyping V2 test | 6, 11, 16, 18, 31, 33, 35, 39, 40, 42, 43, 44, 45, 51, 52, 53, 54, 56, 58, 59, 66, 68, 70 and 74 | 66.1% |
| Africa-2000 [42] | South Africa | KwaZulu-Natal midlands | 52 | Samples obtained with a cervicovaginal rinse. HPV DNA was detected and genotyped with PCR and a HPV 16 specific primer | 16 | 42% |
| Africa-2009 [17] | South Africa | KwaZulu-Natal Midlands | 52 | Samples obtained with a cervicovaginal rinse. HPV DNA was detected with PCR and genotyped through a Roche reverse line blot assay | 6, 11, 16, 18, 26, 31, 33, 35, 39, 40, 42, 44, 45, 51, 52, 53, 54, 55, 56, 58, 59, 66, 68, 73, 82, 83 and 84 | 100% |
| Americas-2009 [9] | Guatemala | Escuintla | 292 | Samples obtained with a cervical brush. HPV DNA was detected and genotyped with PCR and Linear Arrays HPV genotyping test. | 5, 6, 8, 11, 16, 18, 26, 31, 33, 35, 39, 40, 42, 45, 51, 52, 53, 54, 55, 56, 58, 59, 61, 62, 64, 66, 67, 68, 69, 70, 71, 72, 73, 82, 83, 84, CP6108 and IS39 | 67.3% |
| Americas-2001 [27] |
Mexico | Mexico City | 55 | Samples obtained from a gynecological examination. HPV DNA was detected and genotyped with PCR and type specific primers | 3, 6, 11, 16, 18, 31, 33, 34, 35, 39, 53, 56, 58, 59, 66, 68 and 72 | 11.8% |
| Americas -2001 [30] | Mexico | Mexico City | 495 | Samples obtained with a cervical swab. HPV DNA was detected and genotyped with PCR and probe hybridization | 6, 11, 16, 18, 26, 31, 33, 35, 39, 40, 42, 45, 51, 52, 53, 54, 55, 56, 57, 58, 59, 66, 68, 73, 82, and 83 | 48.9% |
| Americas -2012 [18] | Peru | Lima | 199 | Samples obtained with a cervical swab. HPV DNA was detected through PCR and genotyped with Roche Linear Array | 6, 11, 16, 18, 26, 31, 33, 35, 39, 40, 42, 45, 51, 52, 53, 54, 55, 56, 58, 59, 61, 62, 64, 66, 67, 68, 69, 70, 71, 72, 73, 81, 82, 82var, 83, 84, and 89 | 66.8% |
| Americas -2011 [43] | Peru | Lima | 87 | Samples obtained through routine examinations and tested using a linc-blog assay | 6, 8, 11, 16, 18, 26, 31, 33, 35, 39, 40, 42, 45, 51, 52, 53, 54, 55, 56, 58, 59, 61, 62, 66, 67, 68, 69, 71, 72, 73, 81, 83, 84, CP6108 and IS39. | 50.6% |
| Eastern Mediterranean -2006 [26] | Tunisia | Tunis | 64 | Samples obtained with an Ayre spatula. HPV DNA was detected with PCR and genotyped with restriction fragment length polymorphism | 6, 16, 18, 31, 33, 35, 45, 52, 56, 58, 72, 82 and 83 | 43.7% |
| Eastern Mediterranean-2003 [19] | Tunisia | Sousse | 51 | Samples obtained with a cytobrush. HPV DNA was detected and genotyped with PCR and type specific primers | 6, 11, 16, 18, 31, 33, 52 and 58 | 39.0% |
| Europe-2007 [44] | Italy | Campania region | 31 | Samples obtained with a cytobrush. HPV DNA was detected with PCR, direct nucleotide sequenced and identified with BLASTn software | 16, 31, 33, 35, 42, 52, 54, 56, 58, 67, 70 and 81 | 22.6% |
| Europe-2006 [45] | Spain | Madrid and Alicante | 1071 | Samples obtained with a cervical brush. HPV DNA was detected with HPV DNA test Hybrid Capture II and genotyped through PCR | 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 68 | 31.5% |
| Europe-2012 [32] | Spain | Galician | 208 | Samples obtained with a cervical brush. HPV DNA was detected and genotyped with Digene Hybrid Capture II | 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 68 | 7.50-35.5% |
| Europe-2004 [37] | Belgium | Ghent | 93 | Samples were obtained, identified as HPV positive through a reverse hybridization assay and genotyped. | 6, 11, 16, 18, 31, 33, 34, 35, 39, 40, 42, 43, 44, 45, 51, 52, 53, 54, 56, 58, 59, 66, 68, 70, 73 and 74 | 77.4% |
| Europe-2011 [46] | Bulgaria | Sofia | 46 | Samples obtained with a cytobrush. HPV DNA was detected and genotyped through PCR | 6, 11, 16, 18, 31 and 33 | 43.4% |
| Europe-2004 [33] | Spain | Oviedo | 187 | Samples obtained from a gynecological examination. HPV DNA was detected and genotyped through PCR | 6, 11, 16, 18, 31, 33, and 39 | 27.8% |
| Europe-2009 [24] | Spain | Alicante | 549 | Samples obtained with a cervical brush. HPV DNA was detected and genotyped through PCR and Digene Hybrid Capture II | 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 68 | 31% |
| Europe-1997 [47] | Turkey | Ankara | 88 | Samples obtained from a gynecological examination. HPV DNA was detected and genotyped through PCR and type specific primers | 6, 11, 16, 18, 31, 33, 35, 42, 51 and 58 | 2.3% |
| Europe-1993 [20] | The Netherlands | Amsterdam | 44 | Samples obtained with an Ayre spatula or cervical brush. HPV DNA detected and genotyped with PCR and type specific oligonucleotide probes | 6, 11, 16, 18 and 33 | 6.8% |
| Europe-2000 [48] | Denmark | Copenhagen | 182 | Samples obtained through a self administered cervicovaginal lavage kit. HPV DNA was detected and genotyped with PCR and an enzyme immunoassay | 6, 11, 16, 18, 31, 33, 35, 39, 40, 41, 42, 43, 44, 45, 51, 52, 56, 58, 59, 66 and 68 | 32.4% |
| South East Asia-2012 [34] | India | West Bengal | 45 | Samples obtained with a cervical brushes. HPV DNA was detected and genotyped with PCR and type specific PCR primers | 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 68 | 73.3% |
| South East Asia-2012 [21] | Bangladesh | Dhaka | 809 | Samples obtained with a cervical brush. HPV DNA was detected and genotyped with PCR and type specific primers | 16, 18 and 33 | 18.2% |
| South East Asia-2001 [22] | Thailand | Bangkok | 251 | Samples obtained with a cervical swab. HPB DNA was detected and genotyped with PCR based assays | 6, 11, 16, 18, 31, 33, 35, 39 and 45 | 47.0% |
| Western Pacific-2009 [28] | Philippines | Manila | 295 | Samples obtained with a cervical brush. HPV DNA was detected with PCR, cloned and compared with BLAST | 6, 9, 11, 16, 18, 26, 30, 31, 32, 33, 34, 35, 38, 39, 40, 42, 43, 44, 45, 51, 52, 53, 54, 55, 56, 58, 59, 61, 62, 66, 67, 68, 70, 73, 74, 81,82, 83, 84, 86, 87, 90, 91, 102 and JEB2 | 57.2% |
| Western Pacific -2008 [15] | Vietnam | Soc Trang | 282 | Samples obtained through a physical examination and sent to a HPV testing lab | 6, 11,16, 18, 26, 31, 33, 35, 39, 40, 42, 45, 51, 52, 53, 54, 55, 56, 58, 59, 61, 62, 64, 66, 67, 68, 69, 70, 71, 72, 73, 81, 82, 83, 84, IS39 and CP6108, | 85.0% |
| Western Pacific-2008 [35] | Korea | Seoul | 188 | Samples obtained with a cervical swab and cytobrush scrape. HPV DNA was detected and genotyped with PCR and a HPV DNA Chip package | 6, 11, 16, 18, 31, 33, 34, 35, 39, 40, 42, 42, 44, 45, 51, 52, 56, 58, 59, 66, 68 and 69 | 31.9% |
| Western Pacific-2007 [36] | Korea | Seoul | 743 | Samples obtained with a cervical swab. HPV DNA detected and genotyped with an oligonucleotide chip | 6, 11, 16, 18, 31, 33, 34, 35, 39, 40 and 42 | 50.3% |
| Western Pacific-2003 [38] | Korea | 417 | Samples obtained with a cytobrush. HPV DNA was detected through Digene Hybrid Capture II and genotyped through an oligonucleotide microarray | 6, 11, 16, 18, 31, 35, 39, 42, 43, 44, 45, 51, 56, 58, 59 and 68 | 46.5% | |
| Western Pacific-2011 [39) | Japan | Kyoto | 196 | Samples were obtained. HPV DNA was detected with PCR and genotyped with the Kurabo GeneSquare Microarray system. | 6, 11, 16, 18, 30, 31, 33, 34, 35, 39, 40, 42, 45, 51, 53 54, 56, 58, 59, 61, 66 and 68 | 52.6% |
| Western Pacific-2001 (25] | Singapore | Singapore | 187 | Samples obtained with a spatula and cytobrush. HPV DNA was detected and genotyped through PCR | 6b, 16, 18, 31, 33, 34, 35, 45, 56 and 58 | 14.4% |
| Western Pacific-2000 [40] | Singapore | Singapore | 188 | Samples obtained with a cervical scrape. HPV DNA detected and genotyped with PCR and type specific primers | 6b, 16, 18, 31, 33, 34, 35, 45, 56 and 58 | 14.4% |
| Western Pacific-2012 [41] | China | Guangxi | 810 | Samples obtained with a cervical swab. HPV DNA was detected and genotyped with a HPV GenoArray test kit | 6, 11, 16, 18, 31, 33, 35, 39, 42, 43, 44, 45, 51, 52, 53, 56, 58, 59, 66, 68 and CP8304 | 38.9% |
| Western Pacific-2012 [23] | Cambodia | Phnom Penh | 220 | Samples obtained with a cytobrush. HPV DNA was detected and genotyped with PCR and type specific probes. | 6, 11, 16, 18, 26/69, 31, 32/42, 33, 35, 39, 45, 51, 52, 53, 54, 56, 58, 59, 61, 62, 66, 67, 68, 70, 71, 72, 73, 81, 82, 83 and 84 | 41.1% |
Demographic Characteristics
In the papers reviewed, the age range of FSWs was between 15-62 years old. The mean weighted age, as calculated from the mean ages reported and number of sex workers in each study, was 26.9 years. The education level was reported by a few studies and a majority of these FSW had only a primary school education. One study found that FSWs in Vietnam with a secondary or high school education were less likely to be positive for multiple HPV infection types in comparison to women with only a primary school education or no formal schooling [15].
HPV and Regions
The median overall prevalence of HPV in these studies is 42.7%, with a range of 2.3 to 100%. Fig. (2) illustrates the prevalence range of 9 HPV types in the 35 studies. The most highly prevalent HPV types are HPV 6 (11.5%), 16 (38.9%), 18 (23.1%), 31 (28.4%), 33 (25.0%), 39 (21.6%), 51 (25.0%), 52 (32.7%), 56 (24.0%) and 58 (26.0%). Among the studies that also reported multiple infection data, the median prevalence is 20.4%. The most prevalent number of coinfections is 2 (32.1%), 3 (19.4%), 4 (17.9%) and over 5 (10.3%) HPV types.
Fig. (3) illustrates the overall HPV prevalence among FSWs by region. Manuscripts were stratified into WHO defined regions. Twenty-five countries were included in the review, with multiple studies from Belgium, Burkina Faso, Japan, Korea, Mexico, Singapore, South Africa, Spain, Thailand, and Tunisia. The number of tested HPV types ranged from one to over 30 different serotypes. Each study presented in this review tested for HPV 16. Fig. (3) shows the median prevalence of the high risk HPV 16 type by region: Africa (8.0%); Americas (11.1%); Eastern Mediterranean (13.7%); Europe (11.5%); South East Asia: (13.9%); Western Pacific: (6.9%). Prevalence of HPV type 16 ranged from 1.1-38.9% across all regions.
Risk Behaviors
Condom Use and Other Barrier Methods
Consistent condom use with clients varied, with a range of 16%-77% in Africa [16, 17], 65.7-99.2% in the Americas [9,18], 37% in the Eastern Mediterranean [19], 50% in Europe [20], 69.3-82.9% in Southeast Asia [21, 22] and 85-90.8% in the Western Pacific [15,23]. Other popular mechanisms of contraceptives were the barrier method [9] and microbicides [17].
Smoking
Current smoking status varied from 7% in Africa [16], 50.3% in the Americas [9], 83% in the Eastern Mediterranean [19], 44% in Europe [24], and 35% in the Western Pacific [25]. Southeast Asian region studies did not report FSW smoking status.
Alcohol Use
We only have information about alcohol use in studies done in the Eastern Mediterranean region where one study reported all FSW were habitual alcohol users [26], and others reported that 50% currently drank [19].
Age at First Sex
Age at first sexual intercourse for FSWs ranged from 9 [18]-over 20 years old [25]. In all studies the majority of FSWs were less than 20 years old at the age of sexual initiation: Africa 16.7 years old [16]; Americas 9-22 years [18, 27]; Europe: 16 years [24]; and in the Western Pacific 15-20 years of age [15, 23, 28]. Eastern Mediterranean regions did not give a specific age but indicated that a majority were less than 20 years old [19]. Southeast Asian regions did not report an age at first sex.
Time in Sex Work and Partners
Average time in sex work ranged from less than 6 months [27] to 16 years [19]. The number of partners per week ranged from 2 [29] to over 20 [17]. The number of partners per week ranged from 2.3-20 in Africa [17, 29], while 42% of women from studies in the Americas had less than 7 partners [9,30]. Another study from the Americas reported an average of 166 partners in the past month [18], whereas a study from the Western Pacific region reported an average of 18 partners in the past month [15]. One study from Southeast Asia indicated that the majority of women worked less than 20 days per month [22]. A study from Europe reported that 41% of women had less than 1056 partners in the past year [24]. Eastern Mediterranean studies did not report the number of partners.
HPV Sample Types and Detection Methods
All studies included in our review collected samples from cervical or cervico-vaginal swabs, cervico-vaginal lavage kits or from gynecological examinations for HPV detection. HPV testing methods varied between studies and over time, and was independent of geographical region. The most common methods in reviewed studies included line blot hybridization [17], line probe assay [31], hybrid capture 2 [32], linear array [9], RFLP [26], in house PCR [33], and reverse line blot [15].
DISCUSSION
High risk HPV DNA infection is common among FSWs globally, with no clear heightened risk in any geographic location when excluding HIV prevalence. A wide range of sample and testing methodologies over time make it difficult to compare HPV prevalence across any studies. However, the results of this review are indicative of the demographic characteristics of a group of women at high risk for incident HPV infection, the types most common among HPV positive FSWs, and the areas where preventative interventions should be focused for cervical cancer prevention.
One study saw that HPV prevalence was higher in 20-year-old or younger women compared to women over 20 years old [33]. There may be a decrease in HPV type prevalence by age due to clearance of infection and natural immunity, but this may also be due to increased safe sex practices at older ages [49]. While we cannot make this conclusion here from limited data in our review, a decrease in the number of sexual partners with increasing age may also explain this age related patterns of HPV prevalence. Previous studies have shown that this may be attributed to a constant systemic production of HPV antibodies contributing to an immune response against new infection [33].
The prevalence of type specific HPV varied by region. All regions exhibited a high prevalence of the high-risk HPV types 16 and 18 despite differences in HPV detection methods. These results specify the importance of cervical cancer screening in high-risk groups such as FSWs. Other prevalent high-risk types varied by region and were dependent on HPV testing capability for specific genotypes.
HIV is a known risk factor for increased HPV detection and is common among FSWs worldwide [50]. For this reason, we excluded FSWs with HIV infection from the main analysis in our review. We separately analyzed HIV positive women and the prevalence of HPV. The overall prevalence of HPV in HIV positive women is higher than that of HIV negative women. Further reviews warrant analysis of HPV prevalence in HIV positive and HIV negative FSWs for comparison. We also examined various risk factors which are related to HPV infection such as condom use and other barrier methods, smoking, alcohol use, age at first sex, time in sex work and number of partners.
Our review of HPV in FSWs has several limitations. Each study utilized unique laboratory methods in testing multiple HPV types. Additionally, HPV detection methods have markedly improved over time [51]. For example, HPV 16 prevalence has increased when comparing the earliest to the most recent studies in the review, even when testing the same samples at different time periods [17, 42] For this reason, comparing the prevalence of HPV between studies and over time is challenging, as is making conclusions based on data by grouping studies. Potential contamination and limitations of testing only certain genotypes makes it difficult to compare the reported prevalence. Also, some but not all studies evaluated the adequacy of the sample, which might influence HPV DNA detection.
The high HPV prevalence shown among FSWs from 35 studies and 25 countries illustrates the need to prevent chronic infection in high-risk groups. They have a high frequency of abnormal pap smears and are at higher risk of HPV infection due to their exposure to multiple partners in their occupation [10,37]. Both of these factors presumably increase their risk of cervical cancer. They also have a higher prevalence of high-risk types that are associated with cervical cancer. While condoms are effective in decreasing the risk of infection, they do not fully protect against the infection and are not routinely used with FSW clients [52]. Recent clinical trials have shown HPV vaccines to be highly effective against cervical intraepithelial neoplasia (CIN) associated with HPV types 16 and 18 in women who were not infected with these 2 subtypes at the time of immunization [53,54]. Tailoring vaccine interventions that prevent against infection of region-specific HPV subtypes in FSWs may be successful in preventing transmission and cervical cancer cases.
Our findings suggest the need for cervical cancer prevention campaigns especially in developing countries, tailored to high-risk groups such as FSWs. While this population may be difficult to reach, the nature of their job as well as their high prevalence of high-risk serotypes put both themselves and the general population at risk for HPV and cervical cancer. Since few papers present antibody testing among FSWs [55], it is difficult to ascertain the true cumulative exposure to HPV genotypes. However previous studies illustrate antibody prevalence is significantly higher compared to DNA prevalence. While one study has shown acceptability of HPV vaccine in this population, early vaccination before sexual debut is necessary to ensure protection.
Overall we found that FSWs have a high prevalence of HPV, especially high risk subtypes 16 and 18. That being said, the prevalence of HPV identified in this review spans the spectrum from near 0 to 100%, likely dependent on both HPV sampling and testing methods, and risk factors which vary by region. Results of this review highlight the need for tailored intervention programs tailed to FSWs population for prevention of HPV related disease and outcomes.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
Declared none.


