All published articles of this journal are available on ScienceDirect.
Trends in the Treatment of Anemia Using Recombinant Human Erythropoietin in Patients with HIV Infection
Abstract
Background:
Treating anemia with erythropoietin (EPO) to hemoglobin (Hb) endpoints >11 g/dL may increase risk of serious adverse cardiovascular events.
Methods:
We used medical records data (1996-2003 from the Adolescent Spectrum of HIV Disease Project [ASD] and 1996-2006 from the HIV Outpatient Study [HOPS]) to describe EPO prescription patterns for mildly, moderately, or severely anemic HIV-infected patients. We calculated proportions prescribed EPO and treated to Hb>12 g/dL, and tested for trends over time. We calculated median hemoglobin at first EPO prescription, and described temporal changes using linear regression.
Results:
Among 37,395 patients in ASD and 7,005 patients in HOPS, EPO prescription increased over time for moderately anemic patients; for patients with severe anemia, EPO prescription increased only among ASD patients. Hb at EPO prescription decreased over time in ASD patients (median=8.5 g/dL), but not in HOPS patients (median 9.5 g/dL). Percentage of EPO-treated patients with post-treatment Hb>12 g/dL was 18.3% in ASD and stable, and was 56.7% in HOPS and increased over time (p = 0.03).
Conclusions:
Through 2006, EPO prescription increased over time for patients with moderate or severe anemia. Many patients treated with EPO had post-treatment Hb>12 g/dL. Based on 2011 FDA recommendations, changes in previous prescription practices will be needed.
BACKGROUND
Anemia has long been recognized as highly prevalent among untreated patients with HIV infection [1-3]; prevalence of anemia among AIDS patients before HAART is as high as 90% [2]. Anemia has been consistently associated in observational studies with shorter survival [1-3], and prescription of recombinant human erythropoietin (EPO, an erythropoesis stimulating agent, or ESA) has been associated with improved survival in some [1], but not all [4], studies of HIV-infected, anemic patients. Procrit®, an EPO formulation, has a label indication for treatment of AZT-associated anemia in patients with HIV infection [5].
The FDA has issued “black box” warnings in November 2006, and in November 2007 [6], warning of increased risk of thromboembolic events and progression of certain cancers associated with ESA prescription, particularly when ESAs were prescribed to treat to final hemoglobin concentrations (Hb) near physiological levels. Both warnings recommended that patients should not be treated with ESA to hemoglobin levels > 12 g/dL. In June 2011, an even more conservative recommendation was issued by FDA, recommending that ESAs should not be used to increase Hb to concentrations > 11 g/dL [7].
Because Hb endpoint guidelines for treatment of anemia in patients with HIV infection had been based on expert opinion prior to the black box warnings, and because data on EPO prescription patterns in patients with HIV infection have not been reported, the extent to which historical EPO prescription practices of HIV care providers have been in line with the 2007 or 2011 FDA recommendations is unclear, and it is not known if revision of prescription patterns is needed in light of the FDA black box warnings. We sought to describe EPO prescription practices for patients with HIV infection and anemia in the United States from 1996-2006, using data from two large observational cohorts.
PATIENTS AND METHODS
We used data from the Adult and Adolescent Spectrum of HIV Disease Cohort, which collected data on over 60,000 patients in care for HIV infection in over 100 US clinics in 11 US cities from 1990-2004; and the HIV Outpatient Study, which has collected data on over 8,500 patients in care for HIV infection in 12 clinics in 10 US cities from 1992-present. The basic methodology and study sites for these cohorts have been previously described [8-10]. Both cohorts were reviewed and approved by the Institutional Review Board of the Centers for Disease Control and Prevention, and participating study sites. In the case of ASD, a waiver of informed consent was approved by the institutional review board. In the case of HOPS, written informed consent was obtained from all patient participants. In both cohorts, information was collected on hemoglobin measurements, and on prescription of ESAs. In the ASD study, data were collected by retrospective chart review in 6-month abstraction periods; in HOPS, data were collected prospectively at the time of each patient encounter.
To provide context for our analyses of EPO prescription, we assessed the annual prevalence of anemia (defined as at least one Hb < 12 g/dL in women, or < 14g/dL in men) among all patients in whom hemoglobin was measured from 1996-2003 for ASD and from 1996-2006 for HOPS. We tested for trend in prevalence of anemia over these years in each dataset using the Cochran-Armitage test [11].
For patients with anemia, we classified the baseline anemia status as mild (men: 10 g/dL ≤ Hb < 14; women: 10≤ Hb < 12), moderate (8 ≤ Hb < 10), or severe (Hb < 8); according to the National Cancer Institute definitions for severity of anemia, with the mild anemia definition modified to account for different reference ranges by sex [12]. For each severity classification, and for each cohort, we calculated the proportion of anemic patients prescribed EPO by year. We tested for trends in prevalence of treatment over years (1996-2003 for ASD, 1996-2003 and 1996-2006 for HOPS) using the Cochran-Armitage test.
For patients treated with EPO, we calculated the median hemoglobin concentration last measured before treatment, and the median hemoglobin concentration last measured during EPO treatment among patients with follow-up after the initial treatment, by year and cohort. The hemoglobin concentration at the end of therapy was defined as the last measured hemoglobin before the date EPO was discontinued for HOPS patients, and as the hemoglobin in the last abstraction interval during which EPO was prescribed for ASD patients with subsequent follow-up. We tested for linear trend in the hemoglobin concentrations at the end of EPO prescription by least-squares regression, with hemoglobin as the dependent variable and year as the independent variable. We also calculated the proportions of patients who had Hb > 12 g/dL at the end of their course of EPO therapy, by year and cohort. We tested for trend in these proportions using the Cochran-Armitage test.
RESULTS
In the ASD cohort, a total of 37,395 patients contributed 119,906 person-years [PY] of follow time. In the HOPS cohort, 7,005 patients contributed 28,597 PY of follow time. The characteristics of patients observed in the two cohorts differed with respect to race, age, sex, and HIV acquisition mode (Table 1). The overall prevalence of any anemia decreased significantly over time in both cohorts (Fig. 1). For ASD patients, prevalence decreased from 58.5% in 1996 to 52.6% in 2003. For HOPS patients, prevalence decreased from 57.7% in 1996 to 37.5% in 2006.
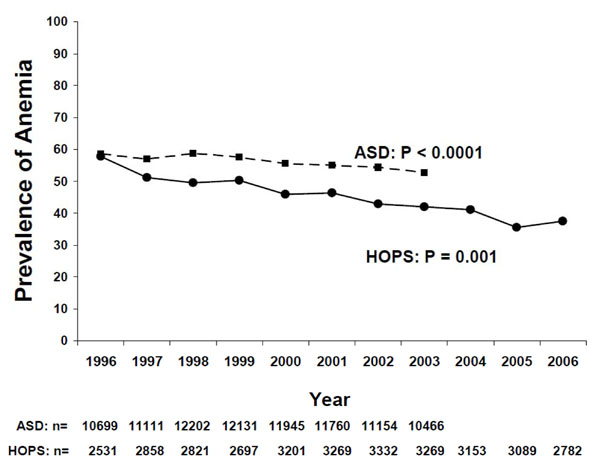
Anemia was defined as a hemoglobin of <12 g/dL for women, and < 14 g/dL for men. P values represent trends in proportions by Cochran-Armitage trend test. N represents number of persons in each study by year.
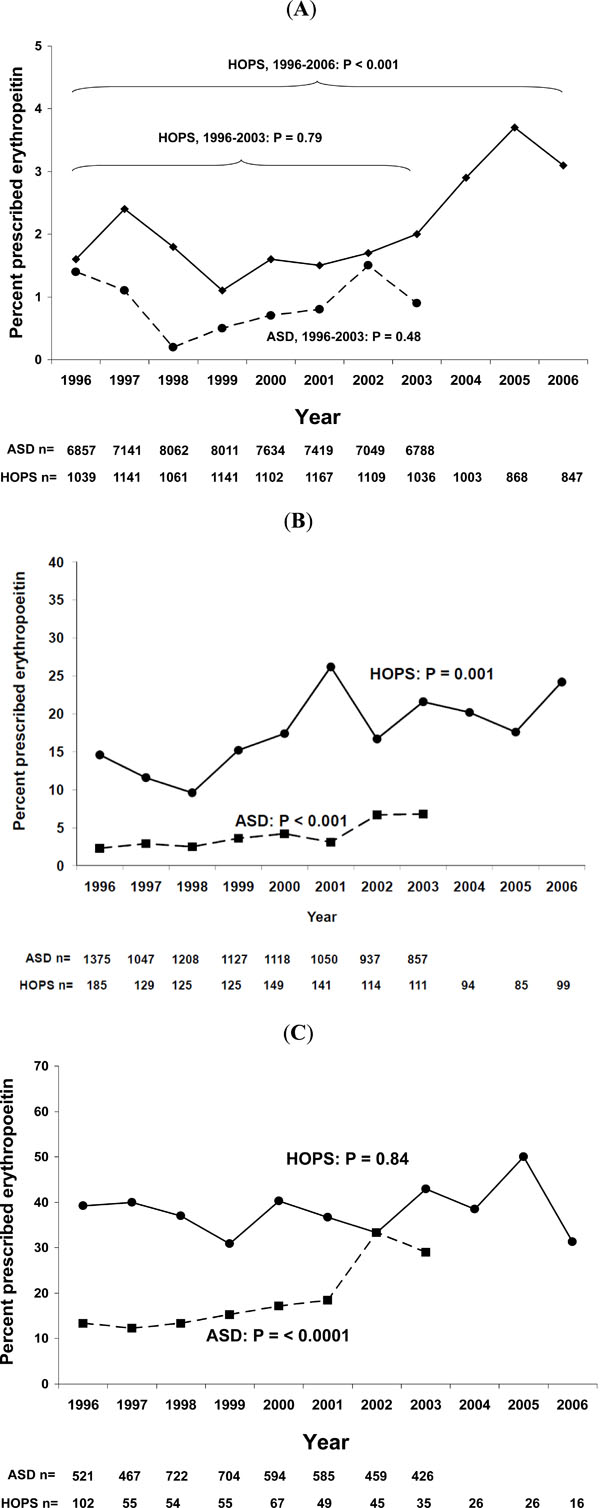
Panel A: Mild anemia was defined as hemoglobin of 10 ≤ Hb <12 g/dL for women, and 10 ≤ Hb <14 g/dL for men. P values represent trends in proportions by Cochran-Armitage trend test. Note limited range of Y-axis scale. N’s represent number of half-person years for ASD, and the number of persons in HOPS. Panel B: Moderate anemia was defined as hemoglobin of 8 ≤ Hb <10 g/dL. P values represent trends in proportions by Cochran-Armitage trend test. Note limited range of Y-axis scale. N’s represent number of half-person years for ASD, and the number of persons in HOPS. Panel C: Mild anemia was defined as hemoglobin of < 8 g/dL. P values represent trends in proportions by Cochran-Armitage trend test. Note limited range of Y-axis scale. N’s represent number of half-person years for ASD, and the number of persons in HOPS.
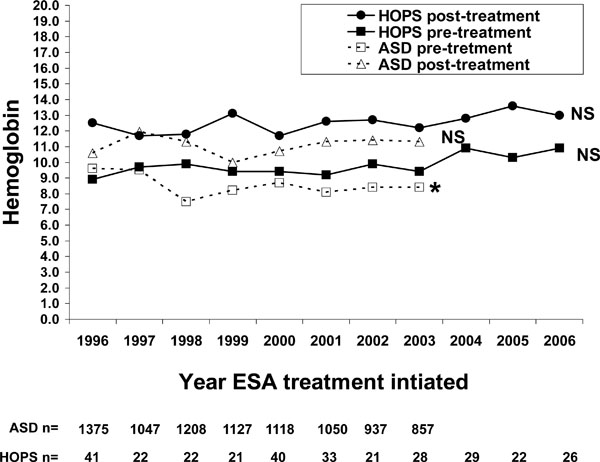
Anemia was defined as hemoglobin of <12 g/dL for women, and < 14 g/dL for men. Symbols represent median values for hemoglobin. Trends in hemoglobin at first prescription or end of prescription, by linear regression modeling hemoglobin = year: results were non-significant (NS: P > 0.05) for all except the trend in ASD pre-treatment hemoglobin concentration (*: p = 0.0002). N’s represent the number of persons prescribed EPO in ASD and HOPS.
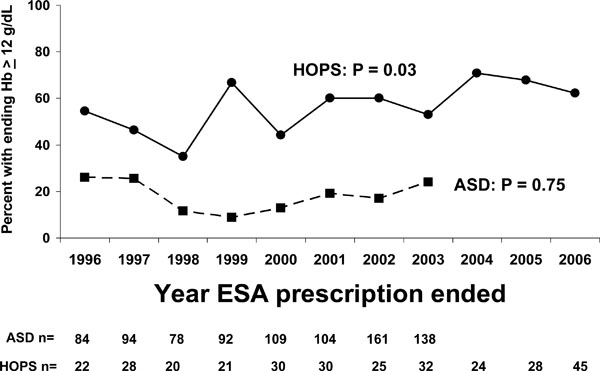
The hemoglobin concentration at the end of therapy was defined as the last measured hemoglobin before the date EPO was discontinued for HOPS patients, and as the hemoglobin in the last abstraction interval during which EPO was prescribed for ASD patients with subsequent follow-up.
Characteristics of Persons with at Least One Hemoglobin Measurement Included in an Analysis of Anemia and Erythropoietin Prescription, Adult and Adolescent Spectrum of HIV Disease Project (ASD), 1996-2003 and HIV Outpatient Study (HOPS), 1996-2006
| Characteristic | ASD | HOPS | ||
|---|---|---|---|---|
| Number of Persons (%) | Person-Years of Observation | Number of Persons (%) | Person-Years of Observation | |
| All | 37,395 (100) | 119,906 | 7005 (100) | 28,597 |
| Race* | ||||
| White, non-Hispanic | 11,493 (31) | 38,013 | 3836 (55) | 16,667 |
| Black, non-Hispanic | 18,067 (48) | 57,325 | 2205 (31) | 8,086 |
| Hispanic | 7,214 (19) | 22,592 | 774 (11) | 3,069 |
| American Indian/Alaskan Native | 354 (1) | 1,130 | 18( <1) | 43 |
| Asian/Pacific Islander | 202 (1) | 720 | 40 (<1) | 183 |
| Other/unknown† | 65 (<1) | 128 | 132 (2) | 549 |
| Age (Years)* | ||||
| 13-29 | 9,395 (25) | 21,677 | 1060 (15) | 3,817 |
| 30-49 | 25,278 (68) | 80,298 | 5238 (75) | 21,794 |
| 50 | 2,722 (7) | 7,932 | 707 (10) | 2,986 |
| Sex* | ||||
| Male | 25,517 (74) | 86,924 | 5579 (80) | 22,759 |
| Female | 9,878 (26) | 32,982 | 1426 (20) | 5,838 |
| HIV Acquisition Mode* | ||||
| Male-male sex (MSM) | 15,126 (40) | 49,675 | 3998 (57) | 17,040 |
| Injection drug use (IDU) | 7,282 (19) | 22,523 | 788 (11) | 2,896 |
| MSM/IDU | 3,027 (8) | 9,856 | 157 (2) | 487 |
| Male-female sex | 5,225 (14) | 18,454 | 1696 (24) | 6,800 |
| Other known risks‡ | 628 (2) | 2,037 | 173 (2) | 701 |
| No reported risk | 6,107 (16) | 17,363 | 193 (3) | 673 |
* Distribution of characteristics differs between ASD and HOPS by χ2 test, p < 0.0001.
† Includes those reporting multiple races, and those reporting single races other than the listed categories.
‡ Includes receipt of transfusion or blood products.
Three primary findings emerged from the analysis of prevalence of prescription of EPO over time. First, prescription of EPO was consistently higher for patients with progressively severe anemia (Fig. 2A-C). For patients with mild anemia (Fig. 2A), prevalence of EPO prescription was lowest (all year-cohort-specific prevalences were < 4%). For patients with moderate anemia (Fig. 2B), year-cohort-specific prevalences of EPO prescription ranged from 2.3%-26%. For patients with severe anemia (Fig. 2C), year-cohort-specific prevalences of EPO prescription ranged from 7.1%-50%.
Second, the prescription of EPO was higher in HOPS patients than in ASD patients. For EPO prescription for patients with mild anemia, HOPS year-specific prevalences ranged from 1.1% to 3.7%; for ASD, year-specific prevalences ranged from 0.1% -0.8%. For EPO prescription for patients with moderate anemia, HOPS year-specific prevalences ranged from 9.6% to 26.2%; for ASD, year-specific prevalences ranged from 2.3% -6.8%. For EPO prescription for patients with severe anemia, HOPS year-specific prevalences ranged from 31.3% to 50.0%; for ASD, year-specific prevalences ranged from 7.1% -20.5%.
Third, EPO prescription increased over time for most cohort-severity specific analyses. EPO prescription increased for mildly anemic patients in HOPS, but only when the entire period from 1996-2006 was considered; when restricting the analysis to the period 1996-2003, no significant trend was observed (Fig. 2A). EPO prescription increased for moderately anemic patients in both cohorts (Fig. 2B). EPO prescription increased for severely anemic patients in ASD, but not for severely anemic patients in HOPS (Fig. 2C).
There was no trend in hemoglobin concentrations at initial EPO prescription in HOPS. In the ASD cohort, the median hemoglobin at first EPO prescription was 9.6 g/dL in 1996 and 8.4 g/dL in 2003, which was a significant decrease over time (Fig. 3). Pre-treatment hemoglobin concentrations in HOPS were higher than in ASD, with year-specific medians ranging from 8.9 g/dL to 10.9 g/dL, but did not change over time. Median post-treatment hemoglobin concentrations were higher in HOPS (range 11.8 g/dL to 13.6 g/dL) than in ASD (range 10 g/dL to 12 g/dL), but hemoglobin concentration at end of prescription did not change over time in either cohort (Fig. 3).
The proportion of anemic patients prescribed EPO who had hemoglobin concentrations > 12 g/dL at the end of their EPO prescription was higher in HOPS (year-specific range, 53%-71%) than ASD (range, 9%-26%). The proportion of patients ending therapy with hemoglobin concentrations > 12 g/dL increased among patients in HOPS from 1996-2006, but did not change among patients in ASD or HOPS from 1996-2003 (Fig. 4).
DISCUSSION
Our data indicate that the EPO prescription practices in diverse US HIV care settings from 1996-2006 were inconsistent with 2007 and 2011 FDA recommendations for duration of ESA therapy; thus, HIV care providers should consider that their earlier EPO prescribing practices may require change in light of FDA black box warnings and recent recommendations about ESAs. According to our data, from 9% to 71% of anemic, HIV-infected patients who received EPO in the several years before the new warnings were issued were treated to hemoglobin concentrations above the currently recommended thresholds. While these patterns of prescription were not inappropriate at the time of our observations, they indicate the need for changes from earlier prescribing practices. To our knowledge, these data are the first detailed published data on EPO prescription practices for anemic patients with HIV infection.
Recommendations on practice strategies and treatment guidelines for use of EPO in patients with anemia and HIV infection were not definitive during the period covered by our report. The label indication for Procrit® in HIV-infected patients was and still is for AZT-related anemia, but research reports [13] and clinical HIV treatment guidelines [14] have broadly recommended consideration of EPO therapy for anemia without identified cause, even when patients are not prescribed AZT. Most anemia in HIV-infected persons in the United States is probably attributable to the anemia of HIV infection (anemia of chronic disease) [4], especially as prescriptions of AZT have decreased in recent years.
Previous recommendations for EPO treatment of anemia have been motivated by a large number of published reports describing the association of anemia and shorter survival (reviewed in 2002 [15]), and by information about quality of life and its relationship to anemia [16]. In 2000, an expert panel concluded that the target hemoglobin for EPO therapy was 12 g/dL for men and 11 g/dL for women [17], but that EPO treatment should be discontinued only if hemoglobin concentration rose to > 13 g/dL during EPO therapy [14, 17]. Thus, for the many patients in our analysis who were treated to hemoglobin concentrations up to 13 g/dL, the EPO prescription practice may have been within recommended practice standards as documented by published treatment recommendations and published expert opinion.
Several new pieces of information have led to a reconsideration of EPO prescription practices, both from the regulatory point of view and based on meta-analytic data about survival and erythropoietin treatment. In 2006, trials of EPO in patients with chronic kidney disease [18, 19] demonstrated no improvement of cardiovascular outcomes, and, in one trial, a higher risk for a composite negative study endpoint (e.g., death or cardiac event). In 2007, preliminary data from an unpublished randomized trial of an ESA in spinal surgery patients found a higher incidence of deep venous thrombosis among EPO-treated patients [20], and several studies reported to FDA have suggested that ESA prescription may be associated with progression of certain cancers, including breast cancer, cervical cancer, and head and neck tumors [20, 21]. Further, a 2007 meta-analysis of data on anemia, survival, and EPO therapy for patients with HIV suggested that treatment with EPO did not improve survival [22]. We did not assess in our analysis endpoints, such as thrombotic events or cancer progression, that are potentially EPO-related, and to the best of our knowledge, there are not data directly demonstrating increased risk for these events associated with EPO prescription in HIV-infected patients.
In other disease conditions, there has been evidence of more aggressive treatment of anemia with ESA in the past 15 years. In a cohort of renal dialysis patients with anemia followed through the United States Renal Data System (USRDS), in 2005 57% of patients were treated with an ESA to hemoglobin concentrations > 13 g/dL; from 1994-2005, mean weekly ESA dose in incident dialysis patients doubled, and the mean monthly hemoglobin concentrations of all patients in care increased by nearly 20% [23]. The increased use of ESA therapy for anemia in renal dialysis patients even caught the attention of the popular press; the New York Times reported that mean dosages of EPO prescribed in the United States were much higher than prescribed doses in other countries, characterizing the US prescribing practices as aggressive [24]. We found that, among patients in our cohorts, the prevalence of EPO prescription was as high as among patients in the USRDS report, and that increases in EPO prescription were mostly observed among patients with moderate or severe anemia. The proportions of anemic HOPS patients treated to hemoglobin concentrations > 12 g/dL from 2003-2006 ranged from 53-71%, similar to the USRDS findings of “overshoot”, suggesting that prescription patterns in the two groups may be similar.
There were consistent differences between HOPS and ASD patients in our outcomes of EPO prescription, and hemoglobin concentrations at treatment initiation and discontinuation. Generally, patients in the ASD cohort were prescribed less EPO, were prescribed EPO at more advanced stages of anemia, and were treated with EPO to lower ending hemoglobin concentrations. Although both ASD and HOPS include a variety of public and private treatment facilities, the facilities included in the ASD cohort are more inclusive of public treatment facilities than are the HOPS treatment settings, and it is likely that some of the differences in EPO prescription between the two cohorts reflect differences in insurance status between patients in the two cohorts. Because the ASD cohort did not collect complete information about insurance status, we could not address this possibility directly.
Our analysis is subject to several limitations. First, despite the large number of diverse clinical settings included in the ASD and HOPS cohorts, the facilities and their patients are not representative of all patients in care for HIV infection in the United States. Also, because our studies were observational, we did not specify when hemoglobin measurements were taken, and the times from the hemoglobin measurements to initiation or discontinuation of EPO prescription were variable. In the ASD cohort, EPO prescription was documented only as occurring within a 6-month interval and in some cases only one hemoglobin measurement may have been recorded per 6 month interval in ASD; this led to less analytic precision than in the HOPS analysis, for which precise dates and length of prescription were abstracted. It is possible that other underlying indications for EPO therapy, such as chronic renal disease, may have increased in the ASD and HOPS patient populations over time, and we did not account for this in our analysis. Also, our datasets did not have additional data, such as MCV, serum EPO concentrations, and other measurements of iron that would have allowed us to classify the likely causes of anemia, so we could not stratify our analysis by etiology of anemia. We also did not analyze data on sequelae or patient well-being as it related to ESA dosing. Finally, our analyses did not span the period in 2006-2007 when the original FDA black box warnings were issued, so we cannot evaluate whether prescription patterns have changed since that time.
Our study documents that from 1996-2006, HIV care providers treated anemia, especially moderate and severe anemia, aggressively with EPO. These changes occurred during a period of time when multiple lines of evidence suggested that managing anemia more aggressively bore clinical consideration, and during which anemic patients (not necessarily HIV-infected) in renal dialysis were treated for anemia with increasing aggressiveness. However, these data taken together with our current understanding of the risks associated with treatment of anemia to hemoglobin concentrations > 11 g/dL in other populations suggest that the EPO prescription patterns of HIV care providers should be substantially more conservative than they were in our historical analysis. Future analyses of observational clinical databases (either cohort or representative cross sectional surveys [25]) may be helpful in documenting changes in EPO prescription practices following the FDA warnings in 2006 and 2007, and the revised FDA recommendations for dosing in 2011.
ACKNOWLEDGEMENTS
Members Of The Adult/Adolescent Spectrum Of HIV Disease Study Group:
Melanie Thompson and Ericka Sinclair (AIDS Research Consortium of Atlanta); David Cohn, Arthur Davidson, and Cornelius Rietmeijer (Denver Department of Health and Hospitals); Jane Turner and Amy Wohl (Los Angeles County Department of Health Services, Los Angeles); Anne Morse, Stephanie Broyles, and C. Lynn Besch (Louisiana Department of Health, Baton Rouge); Eve Mokotoff and Linda Wotring (Michigan Department of Community Health, Detroit); Judy Sackoff and Marie Antoinette Bernard (New York City Department of Health); Jose Otero, Robert Hunter, and Maria de los Angeles Gomez (University Central del Caribe, Bayamon); Sandra Miranda (Puerto Rico Department of Health, San Juan); Susan Buskin, Elizabeth Tesh Barash, and Sharon Hopkins (Public Health–Seattle & King County, Seattle); Sylvia Odem (Texas Department of Health, Austin); Philip Keiser (Parkland Hospital, Dallas); and Kaye Reynolds and Wes McNeely (Department of Health and Human Services, Houston).
DISCLAIMER
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.


