All published articles of this journal are available on ScienceDirect.
Short and Long-Term Incidence of Tuberculosis and CD4-Cell Count Dynamic on HAART in Senegal
Abstract
Objectives: Estimate tuberculosis (TB) incidence among patients receiving HAART. Compare the dynamic of the CD4-cell count and viral load before notification of the TB with the dynamic among patients remaining free of TB.
Design: Prospective cohort with ascertainment of TB cases from medical records.
Methods: The first 404 adults HIV-1 infected patients enrolled in the Senegalese antiretroviral drug access initiative were eligible. CD4-cell and viral load were assessed at baseline and every 6 months. Patients receiving an antituberculosis treatment at HAART initiation were excluded from analysis. Any TB case notified after the first month of HAART was considered as an incident case. Follow-up was censored at death or at the last visit before March 31, 2008. CD4-cell trajectories until TB notification were compared to non-TB developers within two distinct periods: from HAART initiation to 24 months and after.
Results: Over 404 eligible patients, 352 were included in this analysis. Median follow-up reached 73 months and 1821 person-years were accrued. Half of the 42 incident cases were notified before month 19 of HAART yielding to an overall incident rate of 2.3/100 PY [1.7-3.1]. Annual incidence decreased with duration of HAART (trend in incidence: RR=0.26, p<10-4). During the first period, CD4-cell count dynamic of most TB patients was identical to the dynamic among patients remaining free of TB. Most cases of the second period occurred in a context of an immunological failure.
Conclusions: This study provides an estimate of TB incidence among patients on HAART in Senegal and supports two underlying mechanisms.
INTRODUCTION
Tuberculosis (TB) remains the most frequent opportunistic infection and a leading cause of death among patients receiving an antiretroviral treatment (ART) in sub-Saharan Africa, particularly during the first months of treatment, and is even likely to be underestimated [1]. In Senegal, where TB incidence reaches 270 new cases/100 000 population/year (2006), the government has launched an anti-retroviral drug access initiative in 1998 (ISAARV) [2,3]. Among the first adult patients on HAART, the advanced immunodeficiency at treatment initiation, the limited facilities for diagnosis of opportunistic infections and treatment contributed to the high early mortality after HAART initiation. We previously showed that TB was the first single cause of death [4]. In sub-Saharan countries, TB incidence among patients on HAART is usually reported over the first few years of the treatment but the long participation time of the first patients included in the Senegalese initiative provided a unique opportunity to study long-term TB incidence among these patients. In addition, the biological monitoring allowed comparing the CD4-cell count and viral load dynamics between patients developing a TB and the others in order to formulate hypothesis regarding the underlying mechanisms yielding to early or late TB cases.
METHODS
Patients Baseline Characteristics and Initial Antiretroviral Regimen
Detailed inclusion criteria in the Senegalese antiretroviral drug access initiative (ISAARV) and baseline characteristics have been previously described [4]. Patients initially received two nucleoside reverse transcriptase inhibitors combined with either a non-nucleoside reverse transcriptase inhibitors for 58% of them or with an unboosted protease inhibitor in 42% (indinavir except two patients receiving nelfinavir). Only 5% of the patients were non-naïve. Treatment is free of charge since December 2003.
Follow-Up Procedures
After a pre-enrolment and the enrolment visits, patients were re-examined one month after HAART initiation and subsequently every two months unless an adverse event occurred. Every two months visit, a complete clinical examination was performed and every six months, a biological evaluation, including CD4-cell count, viral load assessment and checking of tolerance parameters was carried out. Before the antiretroviral drug prescription, adherence was recorded by the pharmacist.
Every month a meeting was held with the clinical research assistants, the pharmacist and the social workers to trace the patients not showing up at a scheduled visit. After six months without news, the patient was considered as lost to follow-up.
Data Management
Data were recorded from the clinicians on a weekly basis by two clinical research assistants. After a double-keyboard entry using a web-based system (Voozanoo®, EpiConcept, Paris), they were cross-checked for discordance and corrected before export every quarter.
Exclusion Criteria, TB Notification and Case Definition
Patients under anti-TB treatment at HAART initiation and cases occurring during the first month of HAART were excluded. Therefore, only incident cases on HAART, were considered. TB cases could have been declared as an adverse event by the clinician in charge of the patient, specifying a M. tuberculosis infection, pulmonary or extra-pulmonary. Some deaths occurred without a precise diagnosis and the most likely cause of death was then assigned using the post-mortem verbal autopsy procedure we previously used [4].
TB cases were defined as follows:
- Sputum smear or culture-positive pulmonary TB: culture M. tuberculosis + or acid-free bacilli on sputum smear;
- Sputum smear-negative pulmonary TB and extra-pulmonary TB:
- (suggestive clinical presentation) and (chest X-ray or histopathological findings or positive Rivalta reaction on body fluid with predominance of lymphocyte cells) and (response to anti-TB treatment after failure of other ATB treatment).
- post-mortem diagnosis on review of the clinical file completed by a verbal autopsy.
Patients with both pulmonary and extra-pulmonary TB cases were counted as pulmonary TB. Past history of TB was recorded during the pre-enrolment visit.
Data Analysis
Data of non-TB patients were censored at date of death or at last visit before March 31, 2008. Data of TB patients were censored at date of TB notification which could be the date of death if TB diagnosis was done at death only. Annual TB incidence rate on HAART has been estimated using the exact person-time accrued for the specific year.
Dynamic of CD4 cells and viral load before TB notification was examined within two periods, before and after 24 months on HAART, and compared to non-TB patients. A patient with no TB identified within the first period who will subsequently develop a TB during the second period will be considered as a non-TB patient for the first period and therefore serve as control for that period. No CD4-cell counts or VL measured on the month of TB notification or after were used. Missing data were not imputed and Stata 10 was used for all the analysis (College Station, Texas, USA).
Ethical Clearance
The protocol was approved by the Senegal national ethical committee and all the patients gave their written informed consent.
RESULTS
Cohort Characteristics
The first 404 adult patients (221 women), enrolled in Dakar between August 1998 and April 2002, were eligible to the study. At inclusion, 55% of them were at CDC stage C with a median CD4-cell count of 128 [IQR 54 – 217] and a median viral load (VL) of 5.2 log10 [IQR: 4.7 - 5.6]. Cotrimoxazole prophylaxis coverage reached 78% and 28% of the patients had a history of TB.
After exclusion of 47 “prevalent” cases and five cases which occurred during the first month, 352 patients were included in the TB incidence study and followed for a median duration of 73 months [IQR: 28-84] (Fig. 1). As of March 31, 2008, the largest participation time on HAART reached 9.5 years and 1821 person-years of observation were accrued. Between two and seven patients were lost to follow-up per year until the sixth year of the study and 91 all-cause deaths were recorded with a median survival time of 24 months [IQR: 6-: 6-32].
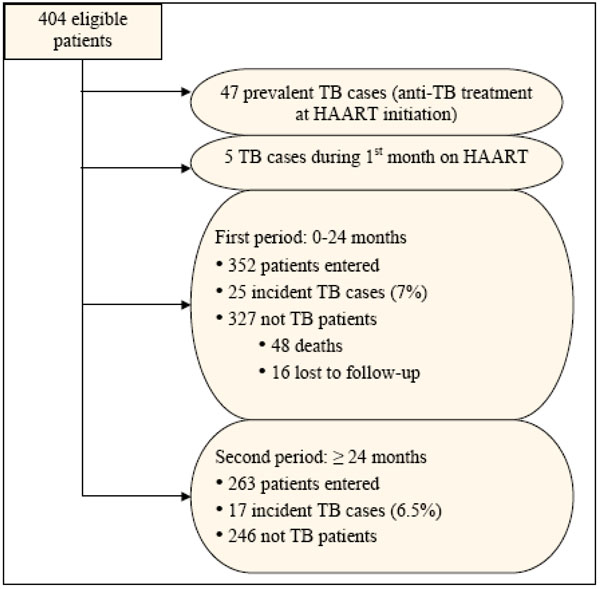
Flowchart of patients selection, ANRS 1215 cohort, Senegal, 1998-2008.
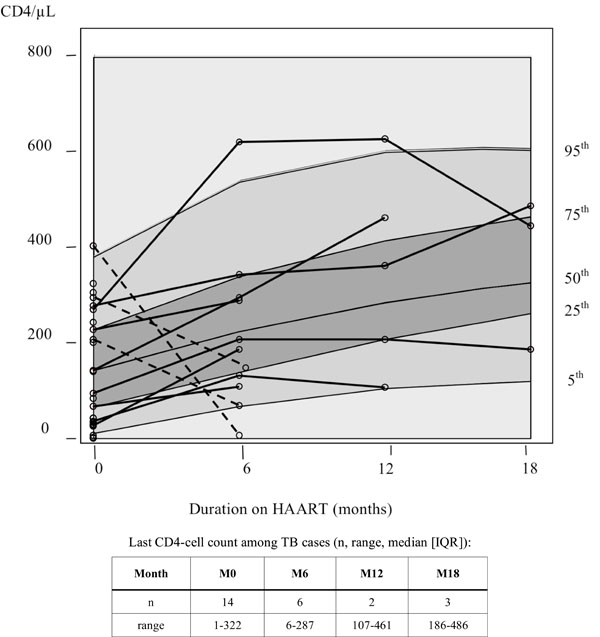
First period: from HAART initiation to 24 months. CD4-cell count trajectories before TB notification (solid lines: positive gain; dashed lines: negative gain) and CD4-cell count progression among TB-free patients as background (solid lines from bottom to top: 5th, 25th, median, 75th and 95th percentile of the distribution).
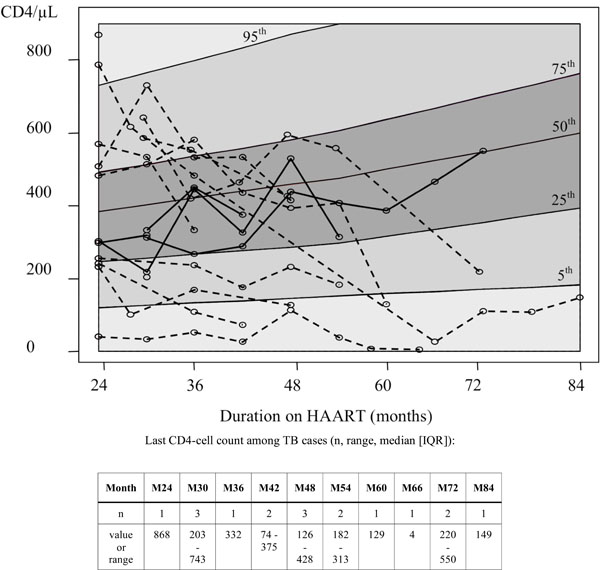
Second period: 24 months and beyond. CD4-cell count trajectories before TB notification (same as Fig. 2).
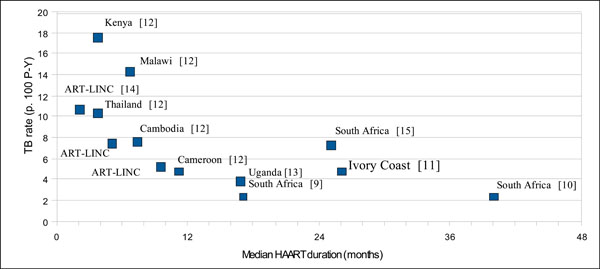
Published estimates of TB incidence on HAART by median follow-up duration.
TB Cases and Incidence Rate
At the date of analysis, 42 incident cases were identified among the 352 patients included in the analysis (12%) with a median notification time of 20 months [IQR: 7 – 51]. Thirteen cases were defined as sputum smear-positive pulmonary TB (31%), 29 as smear-negative of which seven were post-mortem diagnosis (24% of smear-negative). Thirty-four cases were pulmonary TB (81%) and eight extra-pulmonary TB. Nineteen TB patients died (45%), of which 12 (63%) were attributed to TB.
Between initiation of HAART and 24 months (first period), 25 cases were identified and 17 cases after 24 months. The overall incidence rate reached 2.3 cases/100 person-years (P-Y) [95% IC: 1.7 – 3.1]. Over the first 4 years on HAART, the annual incidence rate decreased regularly from 4.5 cases/100 P-Y [95%IC: 2.8 - 7.7] during the first year to 3.5 [1.9 – 6.5] the second year, 1.5 [0.6 - 4.1] the third year and to 0.4/100 P-Y [95%IC: 0.0 - 2.9] the fourth year. After the fourth year, the incidence fluctuated with large confidence intervals around an average rate of 1.8/100 P-Y [95%IC: 0.8-2.9]). The overall test for trend showed that incidence rate decreased from year to year by ¾ on the average (RR=0.26, p<10-4).
CD4-Cell and Viral Load Dynamics
First Period (0-24 Months on HAART)
The main difference laid in the distribution of CD4-cell counts: 40% of the future TB patients were severely immunodepressed, as defined by a CD4 count less than 50 cells/µL, at HAART initiation vs 22% of the TB-free patients (Table 1). However, the difference in median CD4-cell counts did not reach statistical significance. Anaemia at baseline was also statistically more frequent among future TB patients. There was no difference at HAART initiation between the two groups of patients with respect to gender, age, body mass index, cotrimoxazole prophylaxis, viral load or protease inhibitor-containing regimen.
Characteristics of the Patients at HAART Initiation
| Variable | TB (n = 25) | Non TB (n = 327) | Test* |
|---|---|---|---|
| Sexe M | 14 (56%) | 144 (44%) | 0.3 |
| Age median [IQR] | 37 [32 - 42] | 38 [30 - 43] | 0.9 |
| BMI < 19 kg. m-2 | 12 (48%) | 111 (35%) | 0.2 |
| Cotrimoxazole prophylaxis | 22 (88%) | 265 (81%) | 0.6 |
| Hb < 10g/dL | 13 (52%) | 101 (31%) | 0.04 |
| CD4median [IQR]< 5050-199200+ | 95 [33 - 241]10 (40%)5 (20%)10 (40%) | 134 [60 - 218]†70 (22%)150 (47%)101 (31%) | 0.8 0.02 |
| log10 VL median [IQR] | 5.32 [4.75 - 5.59]‡ | 5.17 [4.65 - 5.53]¥ | 0.6 |
| Protease-based regimen (indinavir) | 12 (48%) | 151 (46%) | 0.9 |
* Fisher's exact test or non parametric median test.
† 321 measures;
‡ 21 measures;
¥ 266 measures.
Characteristics of the Patients at 24 Months on HAART
| Variable | TB (n = 17) | Non TB (n = 246) | Test* |
|---|---|---|---|
| Sex M | 10 (59%) | 108 (43%) | 0.3 |
| Age median [IQR] | 38 [31 - 42] | 38 [31 - 44] | 0.6 |
| BMI < 19 kg. m-2 | 2 (18%) | 32 (17%) | 0.9 |
| Cotrimoxazole prophylaxis | 9 (53%) | 95 (39%) | 0.3 |
| Hb < 10g/dL | 3 (18%) | 20 (8%) | 0.2 |
| CD4 median [IQR]M24 measure1st measure available in the period | 303 [241 - 568]¶332 [256 – 587] | 347 [239 - 481]†360 [239 – 521] | 0.70.8 |
| log10 VL median [IQR]M24 measure1st measure available | 2.4 [1.7 – 4.1]‡2.1 [1.7 – 4.6] | 1.7 [1.7 – 2.9]¥1.7 [1.7 – 3.0] | 0.020.01 |
| Undetectable VLM24 measure1st measure available | 3 (25%)‡4 (23%) | 96 (59%)¥142 (58%) | 0.030.009 |
| Protease-based regimen (indinavir) | 6 (35%) | 87 (35%) | 1.0 |
* Fisher’s exact test or non parametric median test
¶ 11 measures
† 149 measures
‡ 149 measures
¥ Fisher’s exact test or non parametric median test
Among the 25 cases identified within the first period, only 11 CD4 trajectories with at least 2 measures before TB notification were available. The background of Fig. (2) displays the 5th, 25th, 50th, 75th and 95th percentile of the CD4-cell progression among patients remaining free of TB over the period and is used as “reference” progression (median 24-month gain : 197 CD4-cells/µL). The 11 CD4 trajectories of TB cases, superimposed on that background, shows that only three trajectories are sharply decreasing, crossing several quintile distribution bands between baseline and 6-month measurement. The other eight courses, all with duration before TB notification beyond six months of HAART, are increasing or comprised within the “reference” progression bands with a median gain of 124 CD4-cells/µL [IQR: 65 – 192] before TB notification.
VL measurements were available for 21 TB cases but, as for the CD4-cell counts, only the baseline VL measurement was available for nine patients and therefore 12 VL trajectories before TB notification could be examined. One TB patient had his viral load increasing, the one with the largest CD4-cell diminution (VL = +1.8 log10 cp/mL; CD4 count = - 398 cells/µL). After exclusion of that patient and, although non-TB and TB patients cannot be compared due to different follow-up durations, the median VL suppression did no differ between the two groups of patients (respectively, -2.73 log10, 266 measures vs -2.75, 11 measures).
Second Period (24 Months and Beyond on HAART)
Among the 327 patients who entered the first period and did not declare a TB, 48 died before 24 months, 16 were lost to follow-up, 263 entered the second period and 17 will develop a TB (Fig. 1). Examination of VL at month-24 of HAART, or at the first available measure in the period, showed that the proportion of undetectable VL was statistically lower among future TB patients of this period compared to the other patients (Table 2). The median VL was also statistically larger among future TB patients. However, the two groups of patients started the period with an equivalent CD4-cell level. None of the other recorded characteristics differed between the two groups.
As for the first period, CD4 cell count evolution of TB patients were drawn against the distribution of CD4-cell progression of non-TB cases (Fig. 3). Among the 17 TB cases identified within this period, 14 CD4 courses with at least 2 measures were available. For the three other patients, only one CD4-cell count was available before TB notification at 24 months (868 cells/µL) or 30 months (203, 743 cells/µL). Among the remaining 14 patients, only four positive progressions with a modest increase, comprised within the interquartile band of CD4-cell reconstitution of non-TB patients, were recorded (median increase in CD4-cell count: +32 [IQR: 15; +141]). The 10 other patients showed a strong deterioration of their immune status before TB notification (median loss in CD4-cell count: -163 [IQR: 354; -93]) which can be seen graphically (Fig. 2). The last CD4 cell count exceeded 500 cells/µL among three patients only (868, 743, 550 cells/µL). On the average, VL did not increase between month-24 on HAART and TB notification (median VL variation = 0 [IQR: -3.3; + 1.2]. The VL until their last visit was also stable among non-TB patients (median VL variation = 0 [IQR: -0.2; +0.6].
DISCUSSION
This study revealed a significant and decreasing trend in TB incidence among patients on HAART from 4.5 TB cases/100 P-Y during the first year of the treatment to 0.4 TB/100 P-Y the fourth year. Subsequently, incidence fluctuated largely around 1.8 cases/100 P-Y due to the small number of cases and persons at risk. The examination of CD4-cell count dynamics before identification of TB cases compared to non TB cases suggested two underlying mechanisms, for early and late cases.
Given the high frequency of sputum smear-negative pulmonary TB in advanced immunodeficient HIV patients, the limited access to mycobacterial culture in the context of our study, and more generally in resource-limited countries, atypical chest X-ray images, unspecific symptoms, our estimates need to be taken with caution [5-7]. Less than a third of the incident cases were sputum smear- or culture-positives (13/42) and 60% (24/42) were diagnosed on clinical or radiological criteria only. An algorithm approach to improve the diagnosis of smear-negative pulmonary and extrapulmonary TB, such as recommended in 2006 by WHO, was not yet implemented [8]. In addition, seven diagnoses were made only by post-mortem verbal autopsy and review of the clinical file. This lack of accuracy in TB diagnosis may have led to false negative as well as false positive cases. However, no change with time in diagnosis facilities happened, preventing a change in misclassification rate with time.
Despite these limitations, our estimates fit with reported TB incidence rates on HAART. Indeed, we have shown that incidence rate is rapidly falling after HAART initiation. This decreasing pattern is universally reported in resource-limited countries [9-15]. Fig. (4) summarizes published estimates as a function of the median duration on HAART. Our yearly estimates, as well as our average estimate rate of 2.3 cases/100 P-Y over a median follow-up duration on HAART of 73 months, fit within this graph. A different picture emerges from a pooled analysis of cohorts from developed countries where TB incidence rate is much lower after three years of HAART: 0.47 case/100 P-Y [16]. The epidemiological setting with a lower TB prevalence in the general population, better TB diagnosis tools and an earlier access to HAART (median baseline CD4-cell count: 280 cells/µL) are likely to explain this difference.
The examination of the immunovirological dynamics before TB notification yields to different hypothesis regarding the underlying mechanisms.
During the early period on HAART, we hypothesis that there was a mixture of mechanisms among the 25 ART-associated TB cases: i) some clinical cases left undiagnosed at HAART initiation due to the lack of sensitive diagnosis tests with a persistent immunodeficiency and ii) subclinical latent cases at HAART initiation with a subsequent immune restoration within the range of the immune restoration of patients remaining free of TB of the same follow-up on HAART, “unmasking” the TB [17-20]. In a context of higher TB incidence, a third of the early cases was attributed to “unmaking” TB [15].
However, the small number of serial CD4-cell count measures during the first year in TB cases precludes distinguishing clearly between mechanisms. Indeed, the constraints in TB diagnosis and biological monitoring in esource-limited settings, the mixture of clinical presentation, the heterogeneity in underlying mechanisms and the difficulty to differentiate between these led to propose grouping together all TB cases occurring after HAART initiation under the term “ART-associated tuberculosis” [21].
Over the second period, after 24 months on HAART, the underlying predominant mechanism was very different. The notification of an incident TB case was almost always preceded by a fall in CD4-cell counts or a small increase, contrasting with a continuing gain in the CD4-cell level among patients remaining free of TB. In addition, a few patients had a persistent immunodeficiency with a CD4-cell count persistently below 200 cells/µL during that period. In South Africa, Lawn et al. showed that a low updated CD4 cell count is associated with TB incidence, even years after HAART initiation and that reaching a CD4 threshold of 500 cells/µL is necessary to minimize TB incidence rate [15]. At the start of this period, future TB patients did not differ from the other patients by their CD4-cell level but their median VL was 10 times greater and 75% of them had a detectable VL compared to 40% in the other patients. These late incident TB cases can be related to a virological failure, followed by an immunological failure, due either a lack of adherence, known to decrease with the duration of HAART in this setting, or the selection of resistance strains without treatment switch [22]. In other words, patients with a detectable VL at 24 months on HAART, even with a good immune restoration, were at risk of developing a TB. For the few TB cases with a last CD4-cells count above 500 cells/µL, an explanation could be a suboptimal restoration of Mycobacterium tuberculosis-specific immune responses and the absence of a protective CD4-cell threshold [23,24].
This study shows that a low baseline CD4-cell count was associated with the occurrence of a TB within the first 2 years on HAART. This effect has already been shown in Cape Town with a median follow-up of 40 months over an equivalent population size but not in Abidjan, perhaps due to a lack of power [11, 25]. Over the second period, only the baseline 24-month VL differed between the two groups of patients, highlighting again the time-dependence of risk factors for clinical events on HAART with immunodeficiency level predicting the early events and VL predicting later events in the course of the follow-up [26]. This underlines the limited access to VL testing and genotyping in resource-limited countries to monitor patients on HAART for several years in order to guide the treatment switch before the occurrence of clinical events.
The high TB incidence on HAART raises the issue of TB screening in all patients at HAART initiation and the isoniazid prevention therapy (IPT) to prevent incident cases [27]. Recommended by WHO in HIV-positive persons with strict programme prerequisites, this policy is seldom implemented in current management of the HIV-TB coinfection [28, 29]. In Brazil and South Africa in a much higher incidence setting, Golub et al., comparing TB incidence rates between cohorts receiving HAART or not and IPT or not, showed that IPT can reduce the risk of TB beyond the reduction due to HAART and call for a wider use of IPT combined with HAART [30,31]. Combined with a screening for TB at baseline and during follow-up of the patient on HAART, especially during the first years, simulation studies shown that this recommendation could greatly reduce TB incidence in high incidence settings, such as South Africa [32]. Recently, the International Union Against Tuberculosis and Lung Diseases and WHO urged for implementing preventive intervention, including IPT [33]. However, given the shortcomings to exclude subclinical TB at baseline and the early high TB incidence on HAART, IPT could be delayed up to a few months on HAART.
CONCLUSION
This study provides long-term estimates of TB incidence on HAART in a sub-Saharan country and confirms that the first months on HAART place the patients at a high risk of developing a TB. It suggests two main mechanisms depending on the duration of HAART, underlying the importance of improving TB screening in the short-term and VL monitoring in the long-term monitoring of patients. The issue of IPT needs to be evaluated with respect to both incidence rates and diagnosis facilities.
ACKNOWLEDGEMENTS
ANRS 1215/90 Study Group
I Ndoye (Conseil national de lutte contre le sida, Dakar, Sénégal), P de Beaudrap, E Delaporte, A Desclaux, JF Etard, B Taverne (UMR 145, Institut de Recherche pour le Développement / Université de Montpellier 1, Montpellier, France), M Basty Fall, A Diouf, C Massidi, A Sarr, L Zié (Centre régional de recherche et de formation sur la prise en charge du VIH/Sida, CHN de Fann, Dakar, Sénégal), I Ndiaye, PS Sow (Service des maladies infectieuses et tropicales, CHN de Fann, Dakar, Sénégal), NF Ngom Guèye (Centre de Traitement Ambulatoire, CHN de Fann, Dakar, Sénégal), K Ba Fall, PM Guèye (Hôpital Principal de Dakar, Sénégal), PA Diaw, H Diop Ndiaye, S Mboup, NC Touré Kane (Laboratoire de virologie-bactériologie, CHN Le Dantec, Dakar, Sénégal), K Diop, B Ndiaye (Pharmacie centrale, CHN de Fann, Dakar, Senegal).
Funding
«Agence Nationale de Recherche sur le Sida et les hépatites virales» (ANRS, France) and «Institut de Recherche pour le Développement» (IRD, France).


