All published articles of this journal are available on ScienceDirect.
RNA Detection and Subtype C Assessment of HIV-1 in Infants with Diarrhea in Ethiopia
Abstract
In the absence of chemoprophylaxis, HIV-1 transmission occurs in 13-42% of infants born to HIV-1 positive mothers. All exposed infants acquire maternal HIV-1 antibodies that persist for up to 15 months, thereby hampering diagnosis. In resource limited settings, clinical symptoms are the indices of established infection against validated laboratorybased markers. Here we enrolled 1200 children hospitalized for diarrheal and other illnesses. 20-25% of those tested, aged 15 months or younger, were found to be HIV-1-seropositive. Where sufficient plasma was available, HIV-1 RNA detection was performed using a subtype-insensitive assay, with 71.1% of seropositive infants presenting with diarrhea showing positive. From sub-typing analysis, we identified that viruses of the C’ sub-cluster were predominated amongst infants. Although this study may overestimate the HIV-1 frequency through testing symptomatic infants, diarrhea can be seen as a useful marker indicating HIV-1 infection in infants less than 15 months old.
INTRODUCTION
The burden of pediatric HIV-1 infection remains high in Africa, where over 90% of the 3 million HIV-1-infected children reside [1]. The vast majority acquire the infection from seropositive mothers either during pregnancy, during labor and delivery or post-partum through breastfeeding [2-5]. Various initiatives are underway in the region to implement short-course antiretroviral regimens for the prevention of mother-to-child-transmission [6, 7]. In the absence of any intervention, transmission occurs in 13-42% of infants [8]. Antibody-based tests for diagnosing perinatally acquired HIV-1 infection have limited value, since maternally acquired antibodies are present in both infected and uninfected infants [9, 10]. Where antigen or viral RNA-based studies are lacking, children are considered infected only, if HIV-1 antibody persists beyond 15 months, or if death occurs from AIDS or an HIV-1-related cause [11].
Laboratory-based diagnosis of HIV-1 infection is not always feasible, and in such circumstances presumptive diagnosis relying on clinical symptoms has been used as an alternative approach [12]. Studies that critically evaluate the association between clinical manifestations presumed to be HIV-1-related and laboratory confirmed diagnosis of HIV-1 infection would help in developing HIV-1 testing algorithms or clinical guidelines to identify symptomatic HIV-1 infection. Indeed, this has long been recognized as a priority need for developing countries, where resources for confirmatory testing are scarce [13].
Here we report on the extent of established HIV-1 infection in a group of HIV-1-seropositive Ethiopian infants, 15 months of age or less. The majority of the infants presented with diarrhea were evaluated for the presence of HIV-1 RNA, as an indicative of established infection. This study also provides an indirect estimate of the predictive value of diarrhea as a marker that flags the presence of HIV-1 infection in Ethiopian infants. We also investigated the virus genotype (subcluster C or C’) in this population of infants living in a setting where HIV-1 subtype C predominates in adults [14-17]. It is the first report on the genotypes infecting children in Ethiopia by utilising a newly developed molecular tool to discriminate between HIV-1 subclusters C and C’, co-circulating in the country.
MATERIALS & METHODS
Selection of Subjects
The samples for this study were drawn from a project originally designed to investigate the etiological agents of childhood diarrhea in Addis Ababa, Ethiopia. This was a cross-sectional study which involved children under 5 years of age who had acute or chronic diarrhea seen at 3 hospitals in Addis Ababa (Tikur Anbessa Referral and Teaching Hospital, Yekatit 12 Hospital and Armed Forces General Hospital), during April 2000 - September 2001. Children presenting to the hospitals with complaints other than diarrhea were included as non-diarrheal controls.
Ethical Approval
The study was approved by the Research and Ethical Clearance Committee of the Ethiopian Health and Nutrition Research Institute, and the National Ethical Clearance Committee at the Ethiopian Science and Technology Commission.
Serology
Within the scope of the original study, plasma from the children was screened for antibodies to HIV-1/HIV-1-2 (Vironostika HIV-1, Uni-Form II plus O, bioMérieux, Boxtel, The Netherlands), and positive results were confirmed by using Western Blot (HIV-1 Blot 2.2, Genelabs Diagnostics, Singapore).
HIV-1 RNA Testing
A subset of HIV-1-seropositive children, aged 15 months and under with diarrhea, were selected for HIV-1 RNA screening using the LTR-based NASBA assay (Primagen, The Netherlands), performed according to the manufacturer’s instructions, and previously shown to have a demonstrated capacity to detect a range of HIV-1 subtypes efficiently [18]. In addition plasma from seropositive children above the age of 24 months was screened for HIV-1 RNA as a positive control for the assay (N=5), as well as plasma from age-matched (15 months or younger) seronegative infants, serving as negative controls (N=5).
Envelope V3-loop Genotyping
HIV-1 RNA available was utilized in the env-based NASBA-molecular beacon assay for the genotype determination described elsewhere [19].
Statistical Analyses
Statistical analyses were performed, using the STATA computer package (Stata Statistical Software, Stata Corporation, College Station, Texas, USA). Analyses were performed using the Wilcoxon rank-sum test.
RESULTS
Serology
Eight hundred and nine children presented with diarrhea and 395 non-diarrheal controls were included in this study. A total of 540 children with diarrhea and 269 non-diarrheal children were tested for the presence of HIV-1 antibodies. A total of 116 (14.3%) children were HIV-1-seropositive, with a median age of 24 months (IQR 12-42), 103 and 13 amongst the diarrheal and non- diarrheal groups, respectively. The difference in HIV-1-seropositivity between the diarrheal and non-diarrheal group (19.1% versus 4.8%) was highly significant (p<0.001).
The age breakdown and percent HIV-1-seropositive per group are presented (Fig. 1). In the diarrheal group, HIV-1-seropositivity was highest (24.5%) in the youngest age category of 0-7 months and there was an overall declining trend in seropositivity with increase in age (p<0.001). In the non-diarrheal controls, the median age was 42 months (IQR 26-51). In the non-diarrheal seropositivity was highest (9.7%) among the children in the age bracket 32-39 months with no significant trend of declining prevalence with age (p=0.54), possibly due to the low numbers of seropositive children in this group.
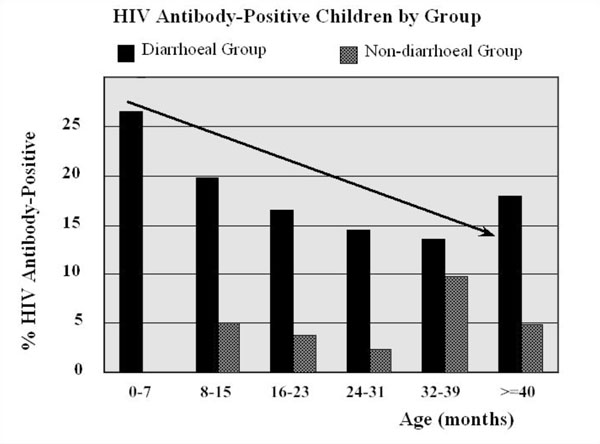
HIV-1 antibody prevalence amongst the study participants in both the diarrheal (n=540) and non-diarrheal groups (n=269). All children were 5 years old or under and were divided in six subgroups according to their age. The arrow indicates the marked initial decline of antibody positivity due to the acquisition of maternal anti-HIV antibodies.
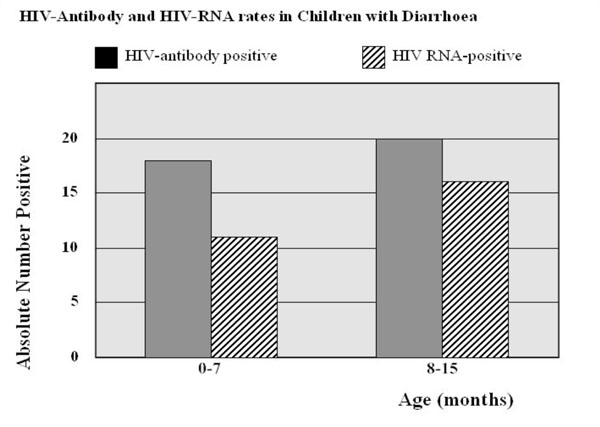
Frequency of detectable HIV-1 RNA in seropositive children with diarrhea needed nosocomial care of 15 months old or younger. The study subjects were divided in two subgroups according to their age.
Detection of HIV-1 RNA, Using the LTR-based NASBA Assay
Amongst the 60 infants aged 15 months or less for the diarrhea group that were HIV-1-seropositive, plasma for HIV-1 RNA testing was available from only 38. Out of these, 27 (71.1%) tested positive for HIV-1 RNA. 11 out of 18 (61.1%) were in the age group of 0-7 months and 16 out of 20 infants (80%) were in the age group of 8-15 months (Fig. 2). In the age-matched non-diarrheal control group, only 1 HIV-1-seropositive child was identified; this infant tested positive for HIV-1 RNA as well. HIV-1 RNA was detected in the plasma of all children older than 24 months that were seropositive but in none of the seronegative children under 15 months of age (data not shown).
Envelope V3-loop Genotyping
The genotype of the V3 domain of the Env protein was determined utilizing a newly developed molecular beacon based NASBA assay, and the 27 diarrheal infants positive for both HIV-1 antibody and RNA were genotyped. Twenty-two could be unambiguously genotyped as subcluster C or C’. Genotype could not be determined for 5 children by the beacon assay, despite the presence of substantial viral load in plasma of the infants. Two of the five samples could be genotyped as cluster C’ by sequencing the C2V3 region of the envelope region. Three of the infants could not be genotyped using either the beacon assay or sequencing. In total nine (37.5%) infants that were infected with a subtype C/subcluster C virus and 15 (62.5%) with a subtype C/sub-cluster C’ virus corroborated adult epidemiological data from both the capital Addis Ababa and the rest of the country [20]. This indicates that transmission rates for the genotypes C and C’ are similar for horizontal and mother to child transmission of HIV-1.
Characteristics of HIV-1 RNA-Positive Infants with Diarrhea
Twenty-seven of 38 HIV-1 seropositive infants (71.1%) of age 15 months or less were positive for HIV-1 RNA and we summarize the results in Table 1. Overall median age was 8 months [IQR 5-12]. Fifteen of the infants were male and 12 were female, with no significant difference in median age by gender: 9 months [IQR 5-12] versus 8 months [IQR 4.5-9] for males and females, respectively (p=0.54). The overall median viral load was 5.0 log10 copies/ml [IQR 4.2-5.6]. The median viral load was slightly higher for females but the difference was not significant (4.6 log10 copies/ml and 5.2 log10 copies/ml for males and females respectively, p=0.150). The median age of C and C’-infected infants was similar (8 months for both subclusters, p=0.509).
Characteristics of HIV-1 RNA-Positive Infants 15 Months and Under with Diarrhoea
|
Median Age by Gender (months) (N=27) Median Viral Load by Gender (log10copies/ml) (N=27) |
Male (N=15) 9 [5-12] 4.6 [3.7-5.6] |
Female (N=12) 8 [4.5-9] 5.2 [4.6-6.0] |
p-value 0.540 0.150 |
|
Median Age by Genotype (months) (N=24) Median Viral Load by Genotype (log10 copies/ml) (N=24) |
C (N=9) 8 [4-12] 4.6 [4.3-5.1] |
C’(N=15) 8 [6-10] 5.2 [4.2-5.8] |
0.509 0.270 |
|
Median Viral Load by Age Category (log10 copies/ml) (N=27) |
0-7 months 5.6 [5.1-6.5] (N=11) |
8-15 months 4.4 [4.0-5.0] (N=16) |
0.020 |
|
Median Viral Load by Age Category & Genotype (log copies10/ml) 0-7 months 8-15 months Median Viral Load by Gender & Age Category (log copies10/ml) Male Female |
C 5.2[4.9-5.6] (N=4) 4.3[4.3-4.5] (N=5) 0-7 months 5.4[4.6-5.9] (N=6) 5.6[5.3-6.5] (N=5) |
C’ 5.6 [5.6-6.5] (N=5) 5.0 [4.1-5.6] (N=10) 8-15 months 4.3 [3.7-4.9] (N=9) 5.0 [4.1-5.6] (N=7) |
0.142 0.391 0.126 0.123 |
Viral load was significantly higher in the younger age category (0-7 months) as compared to the 8-15 months group (5.6 log10 copies/ml [IQR 5.1-6.5] versus 4.4 log10 copies/ml [IQR 4.0-5.0], respectively, p=0.020). When genotype was additionally considered in the analysis, a similar significantly higher difference in viral load was observed in the younger infants (5.2 log10 copies/ml and 4.3 log10 copies/ml for subcluster C, p=0.028, but not for subcluster C’: 5.6 log10 copies/ml and 4.95 log10 copies/ml, p=0.066). However, within age-matched groupings, there was no difference in viral load between the subclusters (5.2 log10 copies/ml and 5.6 log10 copies/ml for C and C’ respectively, in the 0-7 months age group, p=0.142).
In the 8-15 months age category, viral load was 4.3 log10 copies/ml and 5.0 log10 copies/ml for C and C’ respectively,(p=0.391). No significant difference in median viral load was found when comparing different age categories within genders (5.4 log10 copies/ml and 4.3 log10 copies/ml for males in the age categories 0-7 months and 8-15 months respectively, p=0.126). For females viral load was 5.6 log10 copies/ml and 5.0 log10 copies/ml in the two age groups, p=0.123). Similarly no difference was observed in viral load between the genders compared within the same age categories (p=0.465 for the 0-7 months group, and p=0.244 for the age group 8-15 months). Again it should be noted that small sample sizes of the groups may obscure the identification of statistical differences between parameters tested.
DISCUSSION
HIV-1 disease has emerged as the most important cause of morbidity and mortality in children under 5 years of age in many African countries and reversed the improvements in under-five mortality rates achieved in preceding decades [21]. The diagnosis of pediatric HIV-1 infection initially relied on the detection of viral DNA in peripheral blood mononuclear cells using the polymerase chain reaction or virus co-culture techniques [22, 23]. Assays to detect HIV-1 RNA were subsequently developed that helped to characterize HIV-1 infection in children [24, 25], and also served as valuable predictors of disease outcome [26].
Diarrhea as a clinical manifestation of underlying HIV-1 infection in children is well recognized [27-29]. In Africa, diarrhea has been recognized as an important medical complication of HIV-1 infection [30, 31]. Indeed a high proportion (71.1%) of the HIV-1-seropositive infants with diarrhea in our study had detectable HIV-1 RNA in their plasma, indicating established HIV-1 infection. Our results suggest that diarrhea is a major clinical manifestation in HIV-1-infected children in Ethiopia, an observation which concurs with previously reported findings [32-34], although none of these studies utilized HIV-1 RNA detection methods to diagnose infection. Earlier studies did not focus particularly on young infants under 15 months of age, where HIV-1 antibody positivity is not conclusive evidence of an established infection. Taking all children in the study into consideration, there were nearly three times as many seropositive children in the group with diarrhea than in the non-diarrheal group (19.1% versus 4.8%). This finding provides an indirect clue that the presence of diarrhea is a major warning symptom to signal established HIV-1 infection in Ethiopian infants. Thus, where laboratory diagnosis of HIV-1 is not feasible or is limited to antibody detection assays alone, the presence of diarrhea in infants below the age of 15 months may serve as a good proxy indicator of established pediatric HIV-1 infection in Ethiopia.
The source of HIV-1 infection in our series of infants cannot be stated with certainty, although from the age group involved, it can be reasonably assumed that a significant proportion of the infections would have been transmitted from HIV-1-seropositive mothers. Vertical or mother-to-child-transmission has previously been documented to be responsible for 80-90% of HIV-1 infections seen in young children [35], with levels of maternal HIV-1 RNA as an important risk factor [36, 37]. HIV-1 seroprevalence estimates in pregnant Ethiopian women attending antenatal clinics in the capital city Addis Ababa were 15.6% in 2001 [38]. We would expect a similar level of seropositivity in our study which was conducted within approximately the same time frame. However, blood samples were not available from any of the mothers in our study and therefore, their HIV-1 serostatus could not be investigated.
Our study was cross-sectional, limiting us from making broader inferences about the trend of HIV-1-antibody seropositivity over time for a group of 116 seropositive children aged 0-5 years. However, the fact that HIV-1 seroprevalence was highest in the youngest infants and also the declining trend with increasing age, suggests that a substantial fraction of the early seropositivity represents maternally acquired antibodies and not true infection. Alternatively, the fewer numbers of seropositive children in the older age groups could also be a reflection of mortality, since many infected children die at an early age, leaving fewer children in the higher age groups. Mostly infected children develop AIDS and die within the first two years and few survive past the age of 5 years [38]. In our study, of those children infected but surviving beyond the first year of life there was a progressive decline in antibody positivity past the age of 2 years, which was followed again by a slight increase in the 32-39 months age category.
Envelope genotype analysis of the HIV-1 RNA-positive samples using molecular beacons was performed. Most samples were observed to have subcluster C’ sequences which currently predominate in Ethiopia. The proportion of C and C’ isolates was 37.5% and 62.5% respectively, consistent with sequence data from studies among adult Ethiopians, which show a trend of increasing prevalence for subcluster C’ over time. Infants infected with subcluster C’ had higher mean viral loads as compared to subcluster C-infected ones, although this difference is not statistically significant. There was a significant difference in viral load only when comparing the different age categories, being higher in the younger age group. The median ages of C and C’-infected infants were similar, so the higher viral load observed in the C’-infected group was not a function of younger age in this group of children. However, from the studies in HIV-1-seropositive adults being followed longitudinally in Ethiopia, there is an indication of significantly higher viral loads in C’-infected individuals as compared to subcluster C-infected persons who are at a similar stage of infection (manuscript in preparation). The lack of similar finding in the present study may reflect the small sample size.
In summary, the findings reported in this study support the initial hypothesis that diagnosis of diarrhea can be a major clinical symptom of underlying HIV-1 infection in young children. Our study used HIV-1 diagnosis based on the detection of HIV-1 RNA in the infants’ samples, rather than antibody-based assays that are of limited value for children under the age of 15 months. On the other hand, the choice of subjects in our study (hospital-based with diarrhea) may introduce a significant bias due to the selection of an already symptomatic group, risking an overestimation of the extent of established HIV-1 infection in seropositive children in general.
ACKNOWLEDGEMENTS
Samples for this study were collected through the Childhood Diarrheal Diseases Project of the Ethiopian Health & Nutrition Research Institute (EHNRI) Addis Ababa Ethiopia, in collaboration with hospitals in Addis Ababa. Funding for the project was obtained through an in-house grant (EHNRI 9/99). The authors would like to thank all investigators at EHNRI and the other institutions who were involved in the original project design from which samples for this study were drawn. The research reported on here is part of the Ethio-Netherlands AIDS Research Project (ENARP), a collaborative effort of the Ethiopian Health and Nutrition Research Institute (EHNRI), the Amsterdam Municipal Health Service (GG/GD), the Academic Medical Center of the University of Amsterdam (AMC), and the Central Laboratory of the Netherlands Red Cross Blood Transfusion Service (CLB). Financial support for ENARP is through the Netherlands Ministry of Foreign Affairs and the Ethiopian Ministry of Health (MOH) as a bilateral project.


