All published articles of this journal are available on ScienceDirect.
Autoimmune Hepatitis in People Living with HIV: A Case Series and Review of Literature
Abstract
Background:
Autoimmune immune disorders are rare in people living with human immunodeficiency virus (HIV), especially autoimmune-related liver disease. Additionally, diagnosis is challenging as there can be multiple reasons for abnormal liver tests in people living with HIV. Since autoimmune hepatitis responds well to immunosuppression and delaying treatment can be detrimental, its diagnosis and treatment must not be delayed or missed. To increase awareness amongst clinicians, thus ensuring prompt diagnosis and treatment, we present three patients with autoimmune hepatitis in the context of people living with HIV.
Case Presentation:
Three individuals aged between 47-63 years (two females and one male) present with significant transaminitis (alanine aminotransferase 5-10 x upper limit of normal), with two out of the three being autoantibody-positive with an elevated IgG. In all three individuals, a liver biopsy was consistent with autoimmune hepatitis and, in addition, showed advanced hepatic fibrosis/cirrhosis. All individuals presented following immune reconstitution. There was a good response to immunosuppression with a reduction in hepatic fibrosis. All are currently in clinical and biochemical remission.
Conclusion:
Autoimmune hepatitis, though rare, must be considered in the differential diagnosis of abnormal liver tests in people living with human immunodeficiency virus. There should be a low threshold to perform a liver biopsy, which can be diagnostic in the right clinical setting. A prompt institution of immunosuppression is warranted to induce remission and attenuate hepatic fibrosis. However, long-term management and outcomes, including the duration of immunosuppression, remain unclear.
1. INTRODUCTION
Elevated liver enzymes are a common finding in people living with human immunodeficiency virus (HIV) (PLWH), and chronic liver disease is a major cause of morbidity and mortality in this cohort [1]. Immune dysfunction and immunopathogenesis are hallmarks of HIV-related liver disease progression. Hyperactivation of B cells has been implicated in causing an altered autoimmune profile, the most common serum abnormality being polyclonal hypergammaglobulinemia [2]. Reported autoimmune conditions in PLWH include systemic lupus erythematosus, anti-phospholipid syndrome, vasculitis, primary biliary cholangitis, polymyositis, Graves' disease, and idiopathic thrombocytopenic purpura [3].
Autoimmune hepatitis (AIH) is rarely seen in PLWH but has been reported as becoming clinically apparent during immune reconstitution (IRS) following the commencement of antiretroviral treatment (ART) [4]. It has been suggested that this may be because HIV can cause immune dysregulation, leading to pathogenic processes involved in the development of a proinflammatory state, facilitating autoimmune systemic diseases [5]. However, ART, along with treatments for concomitant opportunistic infections, such as antituberculosis medication, can result in drug-induced liver injury (DILI) [6]. Whilst AIH responds well to immunosuppression, DILI may not. Therefore, early diagnosis of AIH is essential; notwithstanding, it can be difficult in the context of HIV, HIV-related comorbidities, and their treatment [7, 8].
We presented three cases of AIH in the setting of HIV and discussed their history and management. We also performed a literature search identifying prior publications assessing AIH in the context of HIV and summarised our findings in the discussion.
1.1. Case 1
A 53-year-old African woman was diagnosed with HIV-1 (subtype C; wild type) in April, 2003. Her CD4 cell count at presentation was 18 cells/mm3 (1%), and HIV RNA was undetectable without treatment (<40 copies/mL). Her past medical history included previously treated tuberculosis. There were no risk factors for liver disease, including excessive alcohol intake, and she was a non-smoker. She was commenced on abacavir, lamivudine, zidovudine, and lopinavir/ritonavir within one month of HIV diagnosis. She then presented with virological failure in 2018, developing dual-class HIV resistance and was switched to tenofovir alafenamide/emtricitabine and darunavir/ritonavir (Fig. 1a). In June, 2020, she was referred to the Joint HIV/Liver clinic for persistently raised alanine aminotransferase (ALT) and aspartate aminotransferase (AST), which were 269iu/L (normal range: 0-33iu/L) and 171iu/L (normal range: 0-32iu/L), respectively. Her CD4 cell count was 283 cells/mm3 (18%), and HIV RNA was undetectable. A viral screen for hepatitis A, B, C and E was negative. Antinuclear antibody (ANA) HE-p2 (nuclear fine-speckled staining) was positive, although no titres were available. Immunoglobulin G (IgG) was elevated at 25.5 g/L (normal range 7-16g/L). Her BMI was 29 with a normal HbA1c (36 mmol/mol) (20 – 41 mmol/mol). Her liver ultrasound (USG) showed a mildly heterogeneous liver and coarse echo texture. Fasting transient elastography (TE) revealed a liver stiffness measurement (LSM) of 7.3 kPa. The pretreatment AIH score was 19 (definite) (revised International Autoimmune Hepatitis Group diagnostic criteria) [9] and 7 (likely) (simplified criteria) [10]. Liver biopsy (June, 2020) was suggestive of AIH, moderate plasma lymphocytic infiltration with mild-moderate interface hepatitis, hepatocyte rosette and emperipolesis, and advanced fibrosis (early cirrhosis). She commenced 30 mg prednisolone (equivalent to 45mg once daily as boosted by darunavir/ritonavir) in July, 2020, resulting in the normalisation of ALT within 2 weeks. Prednisolone was weaned to 5 mg in accordance with the American Association for the Study of Liver Diseases [11] and British Society of Gastroenterology guidelines [12], after which she was commenced on azathioprine 50 mg OD (Fig. 1a). However, in view of neutropenia (which predated azathioprine use), in March, 2021, azathioprine was stopped, and prednisolone 3 mg OD continued (equivalent to 4.5 mg prednisolone due to interaction with ritonavir). At the last follow-up in April, 2023, she remained in clinical and biochemical remission (ALT 14 iu/L, though IgG remained elevated between 21-22 g/L) with LSM improved to 4.1 kPa.

1.2. Case 2
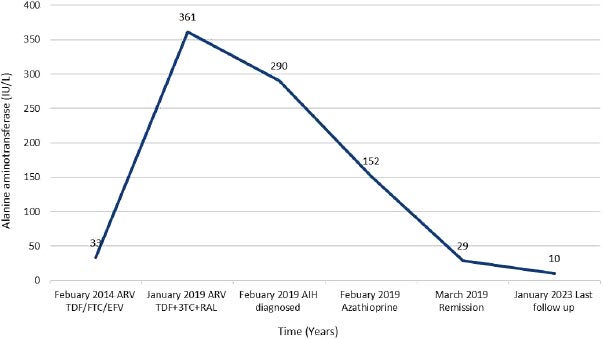
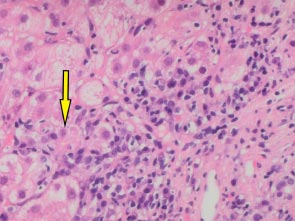
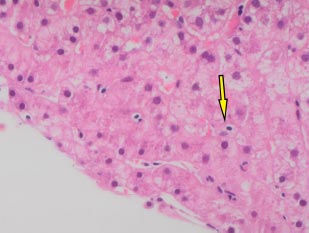
A 66-year-old white man was diagnosed with HIV in December, 2013 (subtype B, wild type). At baseline, his CD4 cell count was 249 cells/mm3 (17%), and his HIV RNA count was 61,517 copies/mL. He had no history of alcohol consumption or illicit drug use and was a non-smoker. ART (tenofovir, emtricitabine, and efavirenz) was started in January, 2014, with HIV RNA becoming undetectable within 6 months (Fig. 1b). In December 2018, he was referred to the Joint HIV/Liver clinic for newly persistently raised ALT 361 (normal range 0-33iu/L) and AST 420 (normal range 0-32iu/L) (Fig. 1b). At referral, the patient was on his third regimen of ART (tenofovir, lamivudine, and rilpivirine), which he had commenced one month previously. A viral serological liver screen was negative. He was ANA positive (1:2,560) with an elevated IgG of 27.0 (normal value 7-16g/L). Liver ultrasound showed a mildly irregular contour of the liver with coarsened echotexture suggestive of cirrhosis, confirmed by a liver stiffness measurement of 33.9 kPa. Liver biopsy (Feb 2019) showed histology consistent with AIH with marked plasma lymphocytic portal inflammation, severe interface hepatitis (Fig. 1c), hepatocyte rosettes and emperipolesis (Fig. 1d), and established cirrhosis. The pretreatment AIH score was 20 (definite) 9 and 8 (likely) [10]. He was commenced on prednisolone 30 mg in February, 2019, with good response, and azathioprine 100 mg once a day started 5 days later. By March, 2019, his liver enzymes normalized (Fig. 1b). However, in May, 2020, upon attempted prednisolone and azathioprine dose reduction, he experienced a biochemical flare with a rise in ALT to 215 iu/L and 247 iu/L in October and December, 2020, respectively, requiring increased doses of both azathioprine and prednisolone. Following this flare, he remained on azathioprine 100 mg and prednisolone 10 mg. LSM improved to 27 kPa by December, 2022. His ALT normalised in June, 2022, and during his last clinic follow-up (Jan 2023), it remained normal at 10 iu/L, and IgG reduced to 18.2 g/L. He remained on azathioprine 100 mg and tapering doses of steroids. His ART changed on two separate occasions during diagnosis and treatment for AIH (Fig. 1b). His ART currently consists of tenofovir, emtricitabine, and raltegravir.
1.3. Case 3
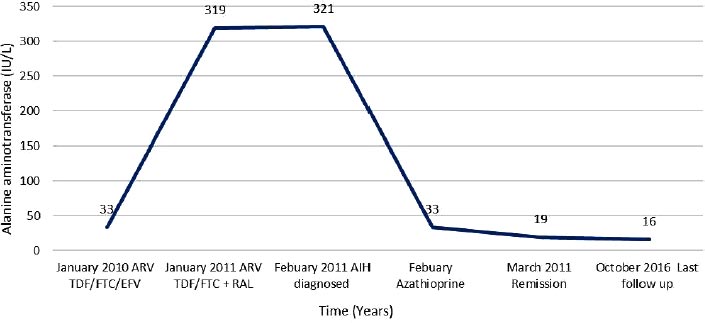
A 47-year-old African woman was diagnosed with HIV in April, 2009, (subtype C; wild type). Her baseline CD4 cell count was 361 cells/mm3 (27%), and HIV RNA was 5,969 copies/m. Her past medical history was unremarkable, with no history of excess alcohol consumption, illicit drug use, opportunistic infections, or smoking. Although diagnosed with HIV in April, 2009, she was commenced on tenofovir, emtricitabine, and efavirenz in January, 2010 [13], and HIV VL was undetectable within 6 months. In January, 2011, she developed a persistently raised ALT of 319 iu/L (0-33iu/L), ALP of 233 (44-147iu/L), and AST of 463 iu/L (0-32iu/L) (Fig. 1e). At the time, efavirenz was changed to raltegravir, her CD4 cell count was 468 cells/mm3 (25%), and HIV RNA was undetectable. Both viral serology for hepatitis and autoantibodies were negative. Imaging (ultrasound, magnetic resonance cholangiopancreatography) was normal. She was subsequently referred to the liver clinic, and a liver biopsy (Feb 2011) was suggestive of AIH, brisk mixed inflammation expanding portal tracts, with good numbers of plasma cells. There were subtle features of cholangiopathy, the significance of which was uncertain. There was moderate portal tract fibrosis with bridging fibrosis present. Though a component of efavirenz DILI could not be excluded, it seemed unlikely, a two-year interval between commencing the drug and the development of abnormal liver tests as well as the presence of bridging fibrosis. She was started on prednisolone 40 mg, which was tapered by 5 mg weekly, followed by 50 mg of azathioprine, which was discontinued due to intolerance. As the patient was not keen to continue prednisolone, this was changed to budesonide 3 mg daily continuing on tenofovir, emtricitabine, and raltegravir. She remained in clinical and biochemical remission, as shown in Fig. (1e). In 2016, she expressed a wish to discontinue budesonide; therefore, a repeat liver biopsy was arranged (June, 2016). This showed significant improvement in hepatic inflammation and fibrosis with on-going portal and minimal interface hepatitis. The patient self-discontinued budesonide in 2016. Liver function tests in October, 2016, were normal, confirming AIH remission. She continues to be monitored by her HIV team.
2. DISCUSSION
We described three cases with AIH in the setting of HIV, all presenting following immune reconstitution. Patients can have advanced liver fibrosis at presentation [11-12], as seen in our three cases, which underscores the need for prompt diagnosis and treatment to induce remission, which prevents disease progression and also attenuates hepatic fibrosis. In all three of our cases, there was an improvement in liver fibrosis after commencing immunosuppression. Delaying treatment can be associated with liver-related morbidity and mortality, especially if ALT at presentation is > 5-10 x the upper limit of normal [11, 12].
AIH is a chronic progressive inflammatory liver disease of unknown aetiology, and the diagnosis rests on a combination of biochemical, immunological, and histological features together with the exclusion of other causes. The diagnostic criteria of AIH include hypergammaglobulinemia, positive serological tests, including ANA and anti-smooth muscle antibodies (ASMA) (seen in 65%-80%), and a characteristic histological appearance, namely interface hepatitis, plasmacytic infiltrate, and regenerative liver-cell rosettes [11, 12]. Diagnosing AIH in PLWH is challenging. Firstly, developing autoimmune disorders in a condition that results in immunosuppression is counterintuitive and may explain the rarity of this diagnosis; a recent literature review demonstrated less than 40 reported cases [14, 15], the first case likely reported approximately 15 years ago [16]. Autoimmune biliary disease in patients with HIV, as potentially reported in our third case, is even rarer [17]. Secondly, both the presence of autoantibodies and hypergammaglobulinemia, considered hallmarks for AIH, is not infrequently seen in PLWH [2, 18]. In fact, autoantibodies have been reported in up to 45% of PLWH [19]. Thirdly, PLWH can have multiple additional reasons for abnormal liver tests, including ART, immune reconstitution, viral hepatitis, and alcohol and non-alcoholic fatty liver disease [20].
Drugs can result in “drug-induced” autoimmune hepatitis (DIAIH) [21-23]. The release of hepatic antigens and consecutive presentation of these autoantigens by immune cells may lead to a continued autoagressive immune reaction in genetically susceptible individuals [22]. The natural history of “drug-induced” AIH is often similar to idiopathic AIH, as such, patients can present with hepatic fibrosis requiring long-term immunosuppression [21-24]. This is in contrast to immune DILI where patients may present with fever, rash, and eosinophilia, and clinically, biochemically, and histologically may be indistinguishable from AIH [24]. Not all individuals with immune DILI need immunosuppression, and those that do, respond well without relapsing upon cessation, unlike that observed in idiopathic and drug-induced AIH.
There is no consistent evidence that ART can cause DILI with an autoimmune phenotype. However, in a recent case series involving 13 patients with HIV and AIH, eight received an efavirenz-based ART, similar to our third case [14]. In the earlier case series, AIH was diagnosed when a patient had immune recovery following ART, consistent with our three cases. This raises the possibility as to whether it is indeed ART-related “drug-induced autoimmune hepatitis” or whether these individuals were always predisposed to develop AIH, which manifested following immune reconstitution. In two large retrospective cohort studies, the prevalence of autoimmune disorders in PLWH was 0.69% -4.1%, with AIH being very uncommon [25, 26]. Most individuals (59%) developed autoimmune disorders months to years following their HIV diagnosis, with approximately 70% receiving ART and having an undetectable HIV viral load [25, 26]. This is consistent with the role played by immune reconstitution. However, there are anecdotal reports of AIH in patients with AIDS-defining illnesses prior to commencing ART, with good response to immunosuppression, despite low CD4 counts [27].
Autoimmune hepatitis is a complex multifactorial polygenic disorder probably caused by the interaction of a trigger and environmental factors in a genetically susceptible individual. Additionally, autoreactivity against liver autoantigens (cytochrome P450IID6; CYP2D6 in type 2 AIH and soluble liver antigen in type 1 AIH) is pivotal in the pathogenesis [28]. Although the exact mechanisms leading to the breakdown of immune tolerance in AIH remain unknown, impairment of immune regulation probably plays a central role. The balance between liver-antigen-specific regulatory T-cells (i.e., CD4posCD25highFOXP3pos) and effector cells sharing specificity for the same autoantigenic regions results in tolerance. If regulatory T-cells are impaired or effector cells are poorly responsive, tolerance to liver autoantigens is lost, leading to the initiation and perpetuation of autoimmune liver damage [28-30]. Antibody-dependent cell-mediated cytotoxic response directed against liver antigens has also been suggested as a causative agent [29]. Several mechanisms in HIV may influence host immunity through immune dysregulation [5, 26]. The virus can promote a pro-inflammatory environment, which overrides the host regulatory networks, thus leading to self-perpetuating autoimmune reactions [31].
Most patients with AIH are women in the 4th to 5th decade, as is also seen in those with HIV and AIH [14]. AIH shows excellent response to immunosuppression and this seems applicable to PLWH. However, in patients with idiopathic AIH, known predictors of poor outcome include the development of cirrhosis during follow-up, failure to achieve and maintain remission, black ethnicity, presence of anti-soluble liver antigen, and presence of DRB3*0101 [32-34]. At present, it is unclear if this would be true in those with AIH and HIV. Additionally, most patients with idiopathic AIH (>70%) will relapse upon cessation of immunosuppression [11, 33, 34] and again this remains unknown in those with AIH in the setting of HIV. In one of our cases, immunosuppression was self-discontinued without a relapse.
CONCLUSION
In conclusion, AIH is a rare disease, and its diagnosis in PLWH remains particularly challenging; nonetheless, it must be considered as a differential diagnosis. This is because AIH shows excellent response to immunosuppression. Timely treatment of AIH attenuates hepatic fibrosis, thus potentially reducing future liver-related morbidity and mortality. However, the long-term management and outcomes of AIH in PLWH, including duration of immunosuppression, remain to be defined, and therefore, regular on-going specialist monitoring is recommended.
LIST OF ABBREVIATIONS
| ALT | = Alanine aminotransferase |
| ANA | = Antinuclear antibody |
| ASMA | = Anti-smooth muscle antibody |
| ART | = Antiretroviral treatment |
| AST | = Aspartate aminotransferase |
| AIH | = Autoimmune hepatitis |
| DILI | = Drug-induced liver injury |
| IRIS | = Immune reconstitution syndrome |
| PLWH | = People living with human immunodeficiency virus |
| TE | = Transient elastography |
| LSM | = Liver stiffness measurement |
ACKNOWLEDGEMENTS
The authors are indebted to the three patients discussed in this case series.


